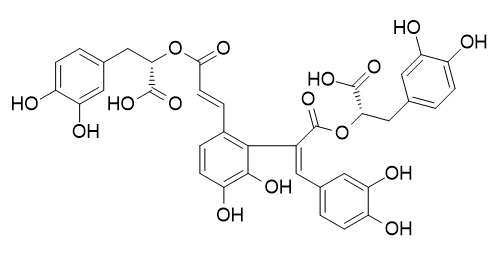
7'(Z)-(8''R,8'''R)-epi-salvianolic acid E
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Korean Med Obes Res.2023, 23:10-7
Molecules.2020, 25(21):5091.
Pharmaceuticals (Basel).2024, 17(4):462.
J Neuroinflammation.2020, 17(1):75.
Biotechnol Bioeng.2020, 117(7):2198-2208.
Molecules.2021, 26(4):816.
Nat Plants.2016, 3:16205
Plant Direct.2021, 5(12):e372.
Proc Biol Sci.2024, 291:20232298.
Food Engineering Progress2019, 23(3)209-216
Related and Featured Products
J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Jun 1;1022:6-12.
Development and validation of a UFLC-MS/MS method for determination of 7'(Z)-(8″S, 8‴S)-epi-salvianolic acid E, (7'R, 8'R, 8″S, 8‴S)-epi-salvianolic acid B and salvianolic acid B in rat plasma and its application to pharmacokinetic studies.[Pubmed:
27064533]
7'(Z)-(8″S, 8‴S)-epi-Salvianolic acid E (compound 1) and (7'R, 8'R, 8″S, 8‴S)-epi-salvianolic acid B (compound 2), two novel analogs of salvianolic acid B (Sal B), have been recently isolated from Salvianolic acid for injection. They both show powerful antioxidant effects, including inducing NQO1 activity and scavenging DPPH free radical, and potential protecting effects for cerebral ischemia. However, no reports have been described the pharmacokinetic study of them.
METHODS AND RESULTS:
In this study, an ultra-fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS) method was developed and validated for the determination of compound 1, compound 2 and Sal B in rat plasma, respectively. Plasma samples were pretreated by liquid-liquid extraction with ethyl acetate. Chromatographic separation was achieved on a Waters Acquity UPLC(®) HSS T3 column (1.7μm particles, 2.1mm i.d.×100mm) with the mobile phase of 0.1% aqueous formic acid (A)-acetonitrile (B) (65:35, v/v). Quantification was performed on a triple quadruple tandem mass spectrometry with electrospray ionization (ESI) by multiple reaction monitoring (MRM) in the negative ion mode. Monitored transitions were set at m/z 717.0→519.0, 717.1→519.1, 717.2→518.9 and 320.9→152.1 for compound 1, compound 2, Sal B and chloramphenicol (internal standard, IS), respectively. Linear calibration curves were acquired over the concentration range of 2.0-1000ng/mL for the three analytes in rat plasma. The extraction recoveries, matrix effects, intra- and inter-day precisions and accuracies of the three analytes were all within acceptable limits. The validated method was successfully applied to the pharmacokinetic study of compound 1, compound 2 and Sal B after intravenous administration of 6.0mg/kg in rats, respectively.
CONCLUSIONS:
The results indicated that compound 1 and compound 2 were both eliminated more slowly than Sal B. Exposure levels of both compound 1 and Sal B were higher than compound 2 in the same dosage range. This study provided critical reference for the pharmacokinetic study of compound 1 and compound 2.