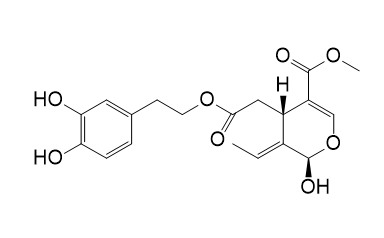
Oleuropein aglycone
Oleuropein aglycone has anti-Alzheimer's disease, anti-breast cancer, anti-inflammatory, anti-hyperglycemic, antioxidant and lipid-lowering properties.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Liq Chromatogr R T2018, 41(12):761-769
Synthetic and Systems Biotechnology2023, j.synbio.
Sci Rep.2018, 8(1)
Nutrients.2023, 15(6):1417.
Neurotoxicology.2022, 91:218-227.
Int J Oncol.2019, 55(1):320-330
Food Funct.2022, doi: 10.1039
Enzyme Microb Technol.2022, 153:109941.
Microchemical Journal2024, 200:110475
Horticulture Research2023, uhad259
Related and Featured Products
Molecules . 2020 Nov 23;25(22):5472.
Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H 2 O 2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway[Pubmed:
33238414]
Oleuropein, a glycosylated secoiridoid present in olive leaves, is known to be an important antioxidant phenolic compound. We studied the antioxidant effect of low doses of Oleuropein aglycone (3,4-DHPEA-EA) and Oleuropein aglycone peracetylated (3,4-DHPEA-EA(P)) in murine C2C12 myocytes treated with hydrogen peroxide (H2O2). Both compounds were used at a concentration of 10 μM and were able to inhibit cell death induced by the H2O2 treatment, with 3,4-DHPEA-EA(P) being more. Under our experimental conditions, H2O2 efficiently induced the phosphorylated-active form of JNK and of its downstream target c-Jun. We demonstrated, by Western blot analysis, that 3,4-DHPEA-EA(P) was efficient in inhibiting the phospho-active form of JNK. This data suggests that the growth arrest and cell death of C2C12 proceeds via the JNK/c-Jun pathway. Moreover, we demonstrated that 3,4-DHPEA-EA(P) affects the myogenesis of C2C12 cells; because MyoD mRNA levels and the differentiation process are restored with 3,4-DHPEA-EA(P) after treatment. Overall, the results indicate that 3,4-DHPEA-EA(P) prevents ROS-mediated degenerative process by functioning as an efficient antioxidant.
Food Chem Toxicol . 2019 Jul;129:1-12.
Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ 1-42 aggregation[Pubmed:
30995514]
Oleuropein aglycone (OleA), the most abundant polyphenol in extra virgin olive oil (EVOO), and Hydroxythyrosol (HT), the OleA main metabolite, have attracted our interest due to their multitarget effects, including the interference with amyloid aggregation path. However, the mechanistic details of their anti-amyloid effect are not known yet. We report here a broad biophysical approach and cell biology techniques that enabled us to characterize the different molecular mechanisms by which OleA and HT modulate the Aβ1-42 fibrillation, a main histopathological feature of Alzheimer's disease (AD). In particular, OleA prevents the growth of toxic Aβ1-42 oligomers and blocks their successive growth into mature fibrils following its interaction with the peptide N-terminus, while HT speeds up harmless fibril formation. Our data demonstrate that, by stabilizing oligomers and fibrils, both polyphenols reduce their seeding activity and aggregate/membrane interaction on human neuroblastoma SH-SY5Y cells. These findings highlight the great potential of EVOO polyphenols and offer the possibility to validate and to optimize their use for possible AD prevention and therapy.
Food Chem Toxicol . 2019 Jul;129:1-12.
Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ 1-42 aggregation[Pubmed:
30995514]
Oleuropein aglycone (OleA), the most abundant polyphenol in extra virgin olive oil (EVOO), and Hydroxythyrosol (HT), the OleA main metabolite, have attracted our interest due to their multitarget effects, including the interference with amyloid aggregation path. However, the mechanistic details of their anti-amyloid effect are not known yet. We report here a broad biophysical approach and cell biology techniques that enabled us to characterize the different molecular mechanisms by which OleA and HT modulate the Aβ1-42 fibrillation, a main histopathological feature of Alzheimer's disease (AD). In particular, OleA prevents the growth of toxic Aβ1-42 oligomers and blocks their successive growth into mature fibrils following its interaction with the peptide N-terminus, while HT speeds up harmless fibril formation. Our data demonstrate that, by stabilizing oligomers and fibrils, both polyphenols reduce their seeding activity and aggregate/membrane interaction on human neuroblastoma SH-SY5Y cells. These findings highlight the great potential of EVOO polyphenols and offer the possibility to validate and to optimize their use for possible AD prevention and therapy.
J Biomol Struct Dyn . 2021 Mar;39(4):1259-1270.
Computational investigation on the effect of Oleuropein aglycone on the α-synuclein aggregation[Pubmed:
32041489]
Parkinson's disease (PD) is considered to be the second most common progressive neurodegenerative brain disorder after Alzheimer's disease, which is caused by misfolding and aggregation of Alpha-synuclein (α-synuclein). It is characterized by distinct aggregated fibrillary form of α-synuclein known as the Lewy bodies and Lewy neurites. The most promising approach to combat PD is to prevent the misfolding and subsequent aggregation of α-synuclein. Recently, Oleuropein aglycone (OleA) has been reported to stabilize the monomeric structure of α-synuclein, subsequently favoring the growth of nontoxic aggregates. Therefore, understanding the conformational dynamics of α-synuclein monomer in the presence of OleA is significant. Here, we have investigated the effect of OleA on the conformational dynamics and the aggregation propensity of α-synuclein using molecular dynamics simulation. From molecular dynamics trajectory analysis, we noticed that when OleA is bound to α-synuclein, the intramolecular distance between non-amyloid-β component domain and C-terminal domain of α-synuclein was increased, whereas long-range hydrophobic interactions between the two region were reduced. Oleuropein aglycone was found to interact with the N-terminal domain of α-synuclein, making this region unavailable for interaction with membranes and lipids for the formation of cellular toxic aggregates. From the binding-free energy analysis, we found binding affinity between α-synuclein and OleA to be indeed high (ΔGbind = -12.56 kcal mol-1 from MM-PBSA and ΔGbind = -27.41 kcal mol-1from MM-GBSA). Our findings in this study thus substantiate the effect of OleA on the structure and stabilization of α-synuclein monomer that subsequently favors the growth of stable and nontoxic aggregates.Communicated by Ramaswamy H. Sarma.
Food Funct . 2017 Dec 13;8(12):4684-4692.
Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives[Pubmed:
29160876]
Oleuropein is the most important phenolic compound present in olive cultivars, but it is scarcely present in extra virgin olive oil (EVOO) due to its high hydrophilicity and degradability. Thus, a set of Oleuropein aglycone derivatives were synthesized by transacetylation under mild conditions with the aim of circumventing these drawbacks and making the active moiety in oleuropein suitable to be added to food fats. The Oleuropein aglycone (closed ring form) is obtained by hydrolyzing oleuropein using Lewis acid catalysis. Then, the permeation profiles as well as the antioxidant capacity of the Oleuropein aglycone derivatives were evaluated by ORAC, DPPH assays and by ROS formation using the SH-SY5Y cell line. The biological activities of the obtained compounds exhibited a dependence on their level of lipophilicity.
J Nutr Biochem . 2017 Feb;40:209-218.
Oleuropein aglycone enhances UCP1 expression in brown adipose tissue in high-fat-diet-induced obese rats by activating β-adrenergic signaling[Pubmed:
27951473]
Oleuropein is the pungent principle of raw olives. Oleuropein aglycone (OA) is a major phenolic compound in extra virgin olive oil and the absorbed form of oleuropein. We aimed to determine the mechanism underlying the nutritional effects of oleuropein and OA on interscapular brown adipose tissue (IBAT) in rats with high-fat (HF) diet-induced obesity by examining the agonistic activity of oleuropein and OA toward the transient receptor potential ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1). Four-week-old male Sprague-Dawley rats were fed an HF (palm oil 30% wt:wt) diet alone or with oleuropein (HF-O, 1 g/kg diet) for 28 days. In rats fed HF-O compared to HF, urinary noradrenaline, adrenaline and UCP1 levels in IBAT were significantly higher, whereas plasma leptin levels and the total weight of the abdominal cavity adipose tissue were significantly lower. In anaesthetized 7-week-old male Sprague-Dawley rats, the OA (3.8 mg of intravenous injection)-induced increase in plasma noradrenaline secretion was suppressed by TRPA1 or TRPV1 antagonist and by a β2- or β3-adrenoceptor antagonist. Furthermore, OA-activated rat and human TRPV1s expressed on HEK293 cells at the same level as zingerone (pungent component in ginger). OA also activated humanTRPA1, and its potency was approximately 10-fold stronger than that for TRPV1. These findings suggest that OA is the agonist of both TRPA1 and TRPV1 and that OA enhances UCP1 expression in IBAT with a concomitant decrease in the visceral fat mass of HF-diet-induced obese rats through enhanced noradrenaline secretion via β-adrenergic action following TRPA1 and TRPV1 activation.
Biochim Biophys Acta Gen Subj . 2018 Jun;1862(6):1432-1442.
Oleuropein aglycone: A polyphenol with different targets against amyloid toxicity[Pubmed:
29571746]
Background: Many data highlight the benefits of the Mediterranean diet and its main lipid component, extra-virgin olive oil (EVOO). EVOO contains many phenolic compounds that have been found effective against several aging- and lifestyle-related diseases, including neurodegeneration. Oleuropein, a phenolic secoiroid glycoside, is the main polyphenol in the olive oil. It has been reported that the aglycone form of Oleuropein (OleA) interferes in vitro and in vivo with amyloid aggregation of a number of proteins/peptides involved in amyloid, particularly neurodegenerative, diseases avoiding the growth of toxic oligomers and displaying protection against cognitive deterioration.
Methods: In this study, we carried out a cellular and biophysical study on the relationships between the effects of OleA on the aggregation and cell interactions of the D76N β2-microglobulin (D76N b2m) variant associated with a familial form of systemic amyloidosis with progressive bowel dysfunction and extensive visceral amyloid deposits.
Results: Our results indicate that OleA protection against D76N b2m cytotoxicity results from i) a modification of the conformational and biophysical properties of its amyloid fibrils; ii) a modification of the cell bilayer surface properties of exposed cells.
Conclusions: This study reveals that OleA remodels not only D76N b2m aggregates but also the cell membrane interfering with the misfolded proteins-cell membrane association, in most cases an early event triggering amyloid-mediated cytotoxicity.
General significance: The data provided in the present article focus on OleA protection, featuring this polyphenol as a promising plant molecule useful against amyloid diseases.
Front Bioeng Biotechnol . 2020 Sep 29;8:908.
Production of Plant-Derived Oleuropein Aglycone by a Combined Membrane Process and Evaluation of Its Breast Anticancer Properties[Pubmed:
33117773]
Natural products and herbal therapies represent a thriving field of research, but methods for the production of plant-derived compounds with a significative biological activity by synthetic methods are required. Conventional commercial production by chemical synthesis or solvent extraction is not yet sustainable and economical because toxic solvents are used, the process involves many steps, and there is generally a low amount of the product produced, which is often mixed with other or similar by-products. For this reason, alternative, sustainable, greener, and more efficient processes are required. Membrane processes are recognized worldwide as green technologies since they promote waste minimization, material diversity, efficient separation, energy saving, process intensification, and integration. This article describes the production, characterization, and utilization of bioactive compounds derived from renewable waste material (olive leaves) as drug candidates in breast cancer (BC) treatment. In particular, an integrated membrane process [composed by a membrane bioreactor (MBR) and a membrane emulsification (ME) system] was developed to produce a purified non-commercially available phytotherapic compound: the Oleuropein aglycone (OLA). This method achieves a 93% conversion of the substrate (oleuropein) and enables the extraction of the compound of interest with 90% efficiency in sustainable conditions. The bioderived compound exercised pro-apoptotic and antiproliferative activities against MDA-MB-231 and Tamoxifen-resistant MCF-7 (MCF-7/TR) cells, suggesting it as a potential agent for the treatment of breast cancer including hormonal resistance therapies.