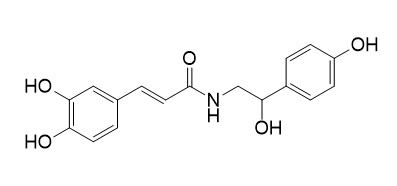
N-trans-caffeoyloctopamine
N-trans-Caffeoyltyramine has both arginase inhibitory property and antioxidant capacity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients2020, 12(3):811.
Chemistry of Natural Compounds2018, 54(3):572-576
Heliyon.2023, e12778.
Biochem Biophys Res Commun.2017, 494(3-4):587-593
Molecules.2018, 23(7):E1659
Int J Mol Sci.2023, 24(14):11496.
Molecules.2019, 24(10):E1926
Agronomy2023, 13(6), 1435.
Ind. J. Pharm. Edu. Res.2023; 57(3):1132-1139.
Pharmacia2024, 71:1-9.
Related and Featured Products
Pmio, 2016, 3(03):e64-e67.
In Vitro Mammalian Arginase Inhibitory and Antioxidant Effects of Amide Derivatives Isolated from the Hempseed Cakes (Cannabis sativa).[Reference:
WebLink]
METHODS AND RESULTS:
In an effort to identify novel inhibitors of arginase, a phytochemical study was performed on hempseed cakes (Cannabis sativa L.). It led to the isolation of a new lignanamide, cannabisin I ( 1), together with seven known lignanamides, cannabisins A, B, C, F, M, 3,3′-demethylgrossamide, grossamide, and two phenylpropanoid amides, N-trans-caffeoyltyramine and N-trans-caffeoyloctopamine, among which was later identified for the first time from C. sativa. Their structures were elucidated by comprehensive analysis of NMR spectroscopy and mass spectrometry data. These compounds were evaluated on mammal arginase (purified liver bovine arginase), showing that N-trans-caffeoyltyramine exhibited the higher activity with an IC50 value of 20.9 µM, which remains, however, less active than the reference compound S-(2-boronoethyl)-l-cysteine (IC50 = 4.3 µM). Radical scavenging capacity of these compounds was determined by the ORAC-FL method.
CONCLUSIONS:
All tested cannabisins displayed antioxidant activity close to or better than the reference compounds. N-trans-Caffeoyltyramine has both arginase inhibitory property and antioxidant capacity.
Biochemical Systematics & Ecology, 2015, 58:265-269.
Phenylpropanoid amides from the roots of Solanum melongena L. (Solanaceae).[Reference:
WebLink]
METHODS AND RESULTS:
Phytochemical investigation of the roots of Solanum melongena L. led to the isolation of 16 phenylpropanoid amides (1–16). Their structures were identified by analysis of spectroscopic data and comparison with those reported in the literature.
CONCLUSIONS:
This is the first report of the natural occurrence of N-trans-sinapoyloctopamine (9) and N-trans-caffeoyloctopamine (10). N-trans-feruloylnoradrenline (12) and N-cis-feruloylnoradrenline (16) were isolated from the genus Solanum for the first time.
Four compounds including 3-(4-hydroxyphenyl)-N-[2-(4-hydroxyphenyl)-2-methoxyethyl] acrylamide (5), 3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)-2-methoxyethyl] acrylamide (6), N-trans-p-coumaroylnoradrenline (11), and N-cis-p-coumaroyloctopamine (15) were firstly reported from S. melongena. The chemotaxonomic significance of these compounds was summarized.