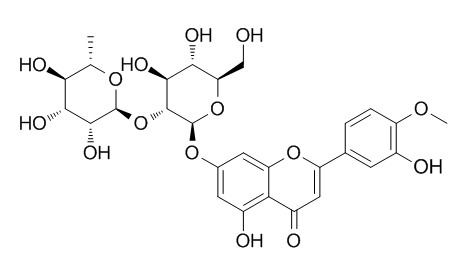
Neodiosmin
Neodiosmin has poor aqueous solubility but exerts a good debittering effect and is a strong antioxidant with potential applications in foods, beverages, and pharmaceutical preparations.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Hepatocell Carcinoma.2022, 9:327-341.
Separation Science Plus2022, sscp.202200048.
J Holistic Integrative Pharm.2023, 4(1):14-28
Int J Mol Sci.2022, 23(13):7115.
Polytechnic University of Catalonia2017, 105826
Int J Mol Sci.2024, 25(18):9909.
Int J Mol Sci.2024, 25(23):12733.
Asian J Beauty Cosmetol2022, 20(2):183-191
Jurnal Ilmu Pertanian Indonesia2023, 28(4):525-533.
Front Pharmacol. 2024, 15:1527494.
Related and Featured Products
J Agric Food Chem. 2013 Feb 27;61(8):1686-93.
HPLC-PDA-MS and NMR characterization of a hydroalcoholic extract of Citrus aurantium L. var. amara peel with antiedematogenic activity.[Pubmed:
22957519]
The phytochemical profile of a hydroalcoholic extract of Citrus aurantium var. amara L. peel, used as herbal medicine, was characterized by HPLC-PDA-MS.
METHODS AND RESULTS:
Two di-C-glycosyl flavones (vincenin II and diosmetin 6,8-di-C-glucoside), a series of flavones (luteolin 7-O-neohesperidoside, rhoifolin, and Neodiosmin), and flavanone (neoeriocitrin, naringin, and neohesperidin) 7-O-neohesperidosides and two methoxyflavones (nobiletin and tangeretin), commonly present in Citrus, were identified. Furthermore, brutieridin and melitidin, two 3-hydroxy-3-methylglutaryl flavanone glycosides, were also characterized along with rhoifolin 4'-glucoside and three coumarins (8,3'-β-D-glucopyranosyloxy-2'-hydroxy-3'-methylbutyl-7-methoxycoumarin, merazin hydrate, and isomerazin). A preparative isolation procedure followed by NMR spectroscopy confirmed the proposed structures of the major flavonoids and identified the coumarins.
CONCLUSIONS:
The phenolic content was found to be 14.8 mg mL(-1), and naringin and neohesperidin were the compounds present in the highest concentration (3.6 and 2.6 mg mL(-1)). The extract of C. aurantium peel inhibited significantly (p < 0.05) both histamine- and dextran-induced edema in rats in a concentration-dependent manner (IC(50) = 119.6 and 118.3 mg kg(-1), respectively), providing evidence for the therapeutic use of C. aurantium var. amara peel.
Starch -Stärke, 2016, 69(5-6):1-9.
Increased solubility and taste masking of a ternary system of neodiosmin with β -cyclodextrin and lysine: Ternary system for increasing solubility and masking bitter tastes[Reference:
WebLink]
Neodiosmin has poor aqueous solubility but exerts a good debittering effect and is a strong antioxidant with potential applications in foods, beverages, and pharmaceutical preparations.
METHODS AND RESULTS:
The water solubility of Neodiosmin was greatly enhanced by forming a Neodiosmin/β-cyclodextrin/lysine ternary inclusion complex. The inclusion type is described in detail in terms of its structural aspects using a phase diagram of solubility. The Neodiosmin/β-cyclodextrin/lysine ternary inclusion complex was synthesized and characterized by thermal analysis and powder X-ray diffractometry (XRD). The molecular and fractal structures of the ternary complexes were investigated using 1H nuclear magnetic resonance (1H NMR) spectroscopy and Fourier transform infrared (FT-IR) spectroscopy. Additionally, scanning electron microscopy (SEM) revealed that Neodiosmin was embedded in a matrix of β-cyclodextrin with lysine.
CONCLUSIONS:
The results demonstrated that the inclusion complex was formed and that the solubility of Neodiosmin was greatly improved. Additionally, the bitterness-masking power of this system was evaluated by a panel test using a series of limonin concentrations as a reference scale. The Neodiosmin/β-cyclodextrin/lysine ternary inclusion complex showed the highest efficacy, and the bitterness attenuation was statistically significant.