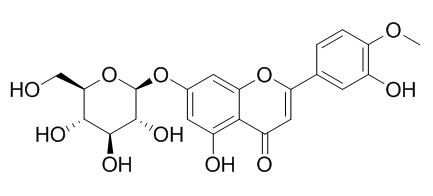
Diosmetin-7-O-beta-D-glucopyranoside
Diosmetin-7-O-beta-D-glucopyranoside has antioxidant activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Sci Food Agric.2022, 102(4):1628-1639
Biomolecules.2024, 14(5):589.
Evid Based Complement Alternat Med.2019, 2019:2135351
J Cell Mol Med.2021, 25(5):2645-2654.
Inflammation.2015, 38(4):1502-16
Sci Adv.2018, 4(10)
Molecules.2023, 28(9):3685.
Molecules.2024, 29(22):5260.
Korean J Pain.2021, 34(4):405-416.
Biomed Pharmacother.2022, 146:112497.
Related and Featured Products
Zuckerindustrie., 2015, 140(10):632-9.
An HPLC-DPPH method for antioxidant activity from sugarcane molasses.[Reference:
WebLink]
Sugarcane molasses is potentially rich in health-promoting phenolic compounds. Present study was designed to optimize experimental conditions for ultrasonic-assisted extraction of antioxidant compounds from sugarcane molasses using response surface methodology.
METHODS AND RESULTS:
An HPLCDPPH method for simultaneous determination of antioxidant activity was also developed. Ethanol concentration 80-84% (v/v), temperature 58-59 degrees C, and 47-50 min time duration were the most favorable conditions. In these conditions, the optimal experimental results were total phenolic content 18 mg gallic acid equivalents/g, with DPPH free radical scavenging ability of 92%, which was same as the predicted values by RSM model. Catechin, vanillic acid, isorhamnetin-3-O-glucoside, eugenol, schaftoside, Diosmetin-7-O-beta-D-glucopyranoside, ferulic acid, and caffeoylquinic acid were identified using HPLC-MS/MS. Among the antioxidant compounds, schaftoside was the most abundant antioxidant (92.081 mu g/g dry substance), while ferulic acid exhibited the highest antioxidant activity.
CONCLUSIONS:
These results suggest that HPLC-DPPH method is more specific, accurate and less time consuming and can sever as a quality control tool.
J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Aug 15;965:150-7.
An efficient preparative procedure for main flavonoids from the peel of Trichosanthes kirilowii Maxim. using polyamide resin followed by semi-preparative high performance liquid chromatography.[Pubmed:
25023212]
In this study, a simple and efficient preparative procedure was developed for preparation of seven flavonoids from the peel of Trichosanthes kirilowii Maxim. using polyamide resin followed by semi-preparative high performance liquid chromatography (SPHPLC).
METHODS AND RESULTS:
First, the ethyl acetate fraction from the peel of T. kirilowii Maxim. obtained "prefractionation" using polyamide resin, which yielded two subfractions. And then the two subfractions were isolated by SPHPLC with an isocratic elution of methanol-water. Finally, seven known flavonoids were purified from 35 g of ethyl acetate extract including quercetin-3-O-[α-l-rhamnose (1→2)-β-d-glucopyranosyl]-5-O-β-d-glucopyranoside (19 mg), quercetin-3-O-rutinoside (24 mg), apigenin-7-O-β-d-glucopyranoside (10mg), diosmetin-7-O-β-d-glucopyranoside (Diosmetin-7-O-beta-D-glucopyranoside,45 mg), luteolin (21 mg), apigenin (15 mg), and diosmetin (56 mg). The purities of the compounds were determined by HPLC and the chemical structures were confirmed by UV and NMR analysis.
CONCLUSIONS:
In the present study, a simple, effective, and rapid procedure was established for preparative separation of multiple components from the peel of T. kirilowii Maxim. Furthermore, it was scalable and economical, so it was a promising basis for large-scale preparation of flavonoids from other plant extracts.