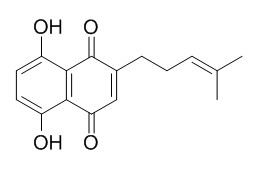
Deoxyshikonin
Deoxyshikonin and dodecyl gallate show significantly synergic antimicrobial activity with penicillin in vivo and in vitro, and can effectively reduce nasopharyngeal and lung colonization caused by different penicillin-resistant pneumococcal serotypes. Deoxyshikonin exerts very good radical scavenging activities toward ABTS+ but shows moderate inhibition of DPPH·, and shows cytotoxic activities. Deoxyshikonin may be a new drug candidate for wound healing and treatment of lymphatic diseases.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2019, 55:229-237
Mol Med Rep.2015, 12(5):7789-95
Life (Basel).2021, 11(12):1399.
Front Plant Sci.2024, 15:1458916.
Korean J. Medicinal Crop Sci.2021, 29(6):425-433
Food Chem X.2024, 21:101208.
Asian J Beauty Cosmetol2022, 20(2):183-191
Curr Issues Mol Biol.2023, 45(3):2136-2156.
Phytomedicine.2022, 102:154183.
J Ethnopharmacol.2024, 324:117775.
Related and Featured Products
Evid Based Complement Alternat Med. 2013;2013:148297.
Enhancement of Lymphangiogenesis In Vitro via the Regulations of HIF-1α Expression and Nuclear Translocation by Deoxyshikonin.[Pubmed:
23737816]
The objectives of this study were to determine the effects of Deoxyshikonin on lymphangiogenesis.
METHODS AND RESULTS:
Deoxyshikonin enhanced the ability of human dermal lymphatic microvascular endothelial cells (HMVEC-dLy) to undergo time-dependent in vitro cord formation. Interestingly, an opposite result was observed in cells treated with shikonin. The increased cord formation ability following Deoxyshikonin treatment correlated with increased VEGF-C mRNA expression to higher levels than seen for VEGF-A and VEGF-D mRNA expression. We also found that Deoxyshikonin regulated cord formation of HMVEC-dLy by increasing the HIF-1 α mRNA level, HIF-1 α protein level, and the accumulation of HIF-1 α in the nucleus. Knockdown of the HIF-1 α gene by transfection with siHIF-1 α decreased VEGF-C mRNA expression and cord formation ability in HMVEC-dLy. Deoxyshikonin treatment could not recover VEGF-C mRNA expression and cord formation ability in HIF-1 α knockdown cells. This indicated that Deoxyshikonin induction of VEGF-C mRNA expression and cord formation in HMVEC-dLy on Matrigel occurred mainly via HIF-1 α regulation. We also found that Deoxyshikonin promoted wound healing in vitro by the induction of HMVEC-dLy migration into the wound gap. This study describes a new effect of Deoxyshikonin, namely, the promotion of cord formation by human endothelial cells via the regulation of HIF-1 α .
CONCLUSIONS:
The findings suggest that Deoxyshikonin may be a new drug candidate for wound healing and treatment of lymphatic diseases.
Front Microbiol. 2015 May 20;6:479.
Antibacterial effects of Traditional Chinese Medicine monomers against Streptococcus pneumoniae via inhibiting pneumococcal histidine kinase (VicK).[Pubmed:
26042111]
Two-component systems (TCSs) have the potential to be an effective target of the antimicrobials, and thus received much attention in recent years. VicK/VicR is one of TCSs in Streptococcus pneumoniae (S. pneumoniae), which is essential for pneumococcal survival. We have previously obtained several Traditional Chinese Medicine monomers using a computer-based screening.
METHODS AND RESULTS:
In this study, either alone or in combination with penicillin, their antimicrobial activities were evaluated based on in vivo and in vitro assays. The results showed that the MICs of 5'-(Methylthio)-5'-deoxyadenosine, octanal 2, 4-dinitrophenylhydrazone, Deoxyshikonin, kavahin, and dodecyl gallate against S. pneumoniae were 37.1, 38.5, 17, 68.5, and 21 μg/mL, respectively. Time-killing assays showed that these compounds elicited bactericidal effects against S. pneumoniae D39 strain, which led to a 6-log reduction in CFU after exposure to compounds at four times of the MIC for 24 h. The five compounds inhibited the growth of Streptococcus pyogenes, Streptococcus mitis, Streptococcus mutans or Streptococcus pseudopneumoniae, meanwhile, Deoxyshikonin and dodecyl gallate displayed strong inhibitory activities against Staphylococcus aureus. These compounds showed no obvious cytotoxicity effects on Vero cells. Survival time of the mice infected by S. pneumoniae strains was prolonged by the treatment with the compounds. Importantly, all of the five compounds exerted antimicrobial effects against multidrug-resistant clinical strains of S. pneumoniae. Moreover, even at sub-MIC concentration, they inhibited cell division and biofilm formation. The five compounds all have enhancement effect on penicillin. Deoxyshikonin and dodecyl gallate showed significantly synergic antimicrobial activity with penicillin in vivo and in vitro, and effectively reduced nasopharyngeal and lung colonization caused by different penicillin-resistant pneumococcal serotypes. In addition, the two compounds also showed synergic antimicrobial activity with erythromycin and tetracycline.
CONCLUSIONS:
Taken together, our results suggest that these novel VicK inhibitors may be promising compounds against the pneumococcus, including penicillin-resistant strains.
Food Chemistry, 2008 , 106 (1) :2-10.
Antioxidants from a Chinese medicinal herb - Lithospermum erythrorhizon[Reference:
WebLink]
Seven compounds, Deoxyshikonin (1), β,β-dimethylacrylshikonin (2), isobutylshikonin (3), shikonin (4), 5,8-dihydroxy-2-(1-methoxy-4-methyl-3-pentenyl)-1,4-naphthalenedione (5), β-sitosterol (6) and a mixture of two caffeic acid esters [7 (7a,7b)] were isolated from Lithospermum erythrorhizon Sieb et. Zucc. and identified by spectroscopic methods. Among them, compound 5 was isolated from this plant species for the first time.
METHODS AND RESULTS:
The antioxidant activities of the seven compounds were compared and evaluated through Rancimat method, reducing power and radical scavenging activity. Results showed that, except compound 6, another 6 compounds all exhibited obvious antioxidant activities against four different methods. Compounds 4 and 7 exerted much more potent antioxidant effects on retarding the lard oxidation than that of BHT and both were found to exhibit strong reducing power. Their antioxidant activities, assessed by Rancimat method and reducing power, decreased in the following order, respectively: compound 7 > 4 > BHT > 2 > 3 > 5 > 1 > 6 and compound 7 > BHT > 4 > 2 approximately 3 approximately 5 > 1> 6. In addition, compounds 1-5 all exerted very good radical scavenging activities toward ABTS+ but showed moderate inhibition of DPPH·, while compound 7 presented as a powerful radical scavenger against both ABTS·+ and DPPH·.
CONCLUSIONS:
Thus, our results suggested that L. erythrorhizon could be a promising rich source of natural antioxidants.
J Ethnopharmacol. 2017 Mar 6;199:128-137.
Wound healing effects of deoxyshikonin isolated from Jawoongo: In vitro and in vivo studies.[Pubmed:
27725239 ]
Jawoongo is a traditional drug ointment (with a traditional botanic formula) used for the treatment of burns and wounds in Korea. One of the components of Jawoongo is Lithospermi Radix (LR, the dried root of Lithospermum erythrorhizon Siebold & Zucc., also known as Zicao or Gromwell), which contains Deoxyshikonin and its derivatives.
The aim of the present study was to investigate the effects of Deoxyshikonin on wound healing.
METHODS AND RESULTS:
The effects of LR extract and Deoxyshikonin on tube formation and migration were measured in human umbilical vein vascular endothelial cells (HUVEC) and HaCaT cells, respectively. We evaluated protein expression of mitogen-activated protein kinase (MAPK) activation by Western blotting. The wound healing effects of Deoxyshikonin was assessed in a mouse model of cutaneous wounds.
The results showed that Deoxyshikonin enhanced tube formation in HUVEC and migration in HaCaT cells. From the western blot analysis, we found that Deoxyshikonin stimulated the phosphorylation of p38 and extracellular signal-regulated kinase (ERK) in HaCaT cells. Moreover, 20μm Deoxyshikonin-treated groups showed accelerated wound closure compared with the controls in a mouse model of cutaneous wounds.
CONCLUSIONS:
In conclusion, the current data indicate that Deoxyshikonin treatment elevated tube formation in HUVECs, and that Deoxyshikonin-induced proliferation and migration in HaCaT cells were mediated by the activation of ERK and p38 MAPKs, respectively. Collectively, these data suggest that Deoxyshikonin in Jawoongo must be an active compound for may be wound healing.
EXCLI J. 2017 Feb 16;16:73-88.
Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem.[Pubmed:
28435429 ]
In this study, the antibacterial and cytotoxic activities of isolated compounds from the roots of Onosma visianii were investigated.
METHODS AND RESULTS:
By using different chromatographic techniques and appropriate spectroscopic methods, the seven naphthoquinones were described: Deoxyshikonin ( 1 ), isobutyrylshikonin ( 2 ), α-methylbutyrylshikonin ( 3 ), acetylshikonin ( 4 ), β-hydroxyisovalerylshikonin ( 5 ), 5,8-O-dimethyl isobutyrylshikonin ( 6 ) and 5,8-O-dimethyl Deoxyshikonin ( 7 ). Among the tested compounds, 3 and 4 exhibited the highest antibacterial activities toward all tested bacterial species (MIC50 and MIC90 for gram positive bacteria: 6.40 μg/mL-12.79 μg/mL and 6.82 μg/mL-13.60 μg/mL, respectively; for gram negative bacteria: 4.27 μg/mL-8.53 μg/mL and 4.77 μg/mL-9.54 μg/mL, respectively). Also, naphthoquinones 3 and 4 exhibited strong cytotoxic activity against MDA-MB-231 cells (IC50 values 86.0 μg/mL and 80.2 μg/mL, respectively), while compounds 1 , 3 , 4 and 5 significantly decreased viability of HCT116 cells (IC50 values of 97.8 μg/mL, 15.2 μg/mL, 24.6 μg/mL and 30.9 μg/mL, respectively).
CONCLUSIONS:
Our results indicated that all tested naphthoquinone pigments are potential candidates for clinical uses as antibacterial and cytotoxic agents.