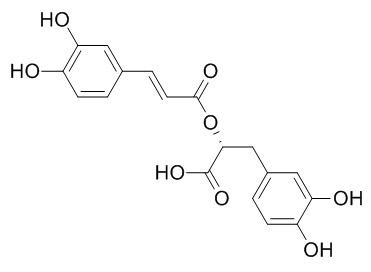
Rosmarinic acid
Rosmarinic acid has antiviral, antibacterial, antiinflammatory, neuroprotective, anticancer, anti-lipid peroxidative, apoptotic,and antioxidant activities. It is used for food preservation, and to treat peptic ulcers, arthritis, cataract, cancer, rheumatoid arthritis, bronchial asthma, and several human neurodegenerative diseases caused by oxidative stress. Rosmarinic acid has the ability to block complement fixation, inhibit lipoxygenase and cyclooxygenase activity and inhibit the expression of CCL11 and CCR3 by suppressing the IKK-β activity in NF-κB activation signaling. It inhibits MAO-A, MAO-B and COMT enzymes with IC50s of 50.1, 184.6 and 26.7 μM, respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2018, 262:78-85
Int J Mol Sci.2018, 19(2)
Evid Based Complement Alternat Med.2018, 2018:1073509
J Nat Prod.2021, 84(9):2544-2553.
bioRxiv - Biochemistry2023, 541790.
Recent Pat Anticancer Drug Discov.2022, 17(4):416-426.
Molecules.2023, 28(13):4907.
Am J Chin Med.2022, 1-20.
Evid Based Complement Alternat Med.2017, 2017:1401279
Metabolites2023, 13(1), 3.
Related and Featured Products
Phytomedicine. 2015 Jan 15;22(1):213-22.
Epigallocatechin gallate, ellagic acid, and rosmarinic acid perturb dNTP pools and inhibit de novo DNA synthesis and proliferation of human HL-60 promyelocytic leukemia cells: Synergism with arabinofuranosylcytosine.[Pubmed:
25636891]
Epigallocatechin gallate (EGCG), ellagic acid (EA) and Rosmarinic acid (RA) are natural polyphenols exerting cancer chemopreventive effects. Ribonucleotide reductase (RR; EC 1.17.4.1) converts ribonucleoside diphosphates into deoxyribonucleoside diphosphates being essential for DNA replication, which is why the enzyme is considered an excellent target for anticancer therapy.
METHODS AND RESULTS:
EGCG, EA, and RA dose-dependently inhibited the growth of human HL-60 promyelocytic leukemia cells, exerted strong free radical scavenging potential, and significantly imbalanced nuclear deoxyribonucleoside triphosphate (dNTP) concentrations without distinctly affecting the protein levels of RR subunits (R1, R2, p53R2). Incorporation of (14)C-cytidine into nascent DNA of tumor cells was also significantly lowered, being equivalent to an inhibition of DNA synthesis. Consequently, treatment with EGCG and RA attenuated cells in the G0/G1 phase of the cell cycle, finally resulting in a pronounced induction of apoptosis. Sequential combination of EA and RA with the first-line antileukemic agent arabinofuranosylcytosine (AraC) synergistically potentiated the antiproliferative effect of AraC, whereas EGCG plus AraC yielded additive effects.
CONCLUSIONS:
Taken together, we show for the first time that EGCG, EA, and RA perturbed dNTP levels and inhibited cell proliferation in human HL-60 promyelocytic leukemia cells, with EGCG and RA causing a pronounced induction of apoptosis. Due to these effects and synergism with AraC, these food ingredients deserve further preclinical and in vivo testing as inhibitors of leukemic cell proliferation.
Life Sci. 2014 Sep 15;113(1-2):7-13.
Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells.[Pubmed:
25058919 ]
Oxidative stress plays a key role in several ailments including neurodegenerative conditions. The aim of the study was to demonstrate the effect of Rosmarinic acid (RA) in preventing oxidative stress related death of neuronal cell lines.
METHODS AND RESULTS:
In the present study, we demonstrated direct neuroprotective effect of RA using H2O2-induced oxidative challenge in N2A mouse neuroblastoma cells. The mechanism of neutralization of H2O2-induced toxicity by RA was evaluated using MTT, lactate dehydrogenase, mitochondrial membrane potential (MMP), intracellular ROS, and comet assays. Up-regulation of brain neuronal markers at molecular level was performed by RT-PCR.
Results presented in the paper indicate that H2O2-induced cytotoxicity in N2A cells was suppressed by treatment with RA. Moreover, RA is very effective in attenuating the disruption of lactate dehydrogenase, mitochondrial membrane potential and intracellular ROS. Pretreatment with RA significantly prevents genotoxicity (3.7-fold, p<0.01) and promotes the up-regulation of tyrosine hydroxylase (TH) (4.5-fold, p<0.01), and brain-derived neurotrophic factor (BDNF) genes (5.4-fold, p<0.01) against H2O2-induced cytotoxicity in N2A cells.
CONCLUSIONS:
Our results revealed that N2A cells are suitable cellular models to evaluate neuroprotective effects of RA, and suggest that RA may potentially serve as an agent for prevention of several human neurodegenerative diseases caused by oxidative stress.
Biochem Pharmacol. 2007 Oct 1;74(7):960-8.
Rosmarinic acid induces melanogenesis through protein kinase A activation signaling.[Pubmed:
17651699 ]
Melanogenesis is a physiological process that results in the synthesis of melanin pigments, which play a crucial protective role against skin photocarcinogenesis.
METHODS AND RESULTS:
In order to determine the effects of Rosmarinic acid on melanogenesis and elucidate the molecular events of melanogenesis induced by Rosmarinic acid, several experiments were performed in B16 melanoma cells. In this study, we showed that the melanin content and tyrosinase expression were increased by Rosmarinic acid in a concentration-dependent manner. In addition, after the melanin content was increased by Rosmarinic acid, it was reduced by H-89 and KT 5720, protein kinase A (PKA) inhibitors, but not by SB203580, a p38(mapk) inhibitor, or Ro-32-0432, a PKC inhibitor, which suggests the involvement of PKA in Rosmarinic acid-induced melanogenesis. Consistent with this, Rosmarinic acid induced the phosphorylation of CRE-binding protein (CREB), but had no effect on the phosphorylation of p38(mapk) or the inhibition of Akt phosphorylation. Additionally, Rosmarinic acid induced the activation of cAMP response element (CRE) without having any effect on cAMP production, which suggests that Rosmarinic acid-induced melanogenesis is mediated by PKA, which occurs downstream of cAMP production. This result was further confirmed by the fact that Rosmarinic acid-induced phosphorylation of CREB was inhibited by H-89, but not by PD98059, a MEK1 inhibitor, or by LY294002, a phosphatidylinositol-3-kinase (PI3K) inhibitor. Rosmarinic acid-induced expression of tyrosinase protein was attenuated by H-89.
CONCLUSIONS:
Based on these results, we report for the first time that Rosmarinic acid induces melanogenesis through PKA activation signaling.
Br J Pharmacol. 2006 Jun;148(3):366-75.
Rosmarinic acid as a downstream inhibitor of IKK-beta in TNF-alpha-induced upregulation of CCL11 and CCR3.[Pubmed:
16604092 ]
1. Tumor necrosis factor (TNF)-alpha is known to induce the expression of CCL11 and CCR3 via the activation of NF-kappaB. CCL11 (eotaxin), the C-C chemokine, is a potent chemoattractant for eosinophils and Th2 lymphocytes, and CCR3 is the receptor for CCL11.
METHODS AND RESULTS:
2. In order to determine the effects of Rosmarinic acid on the TNF-alpha-induced upregulation of CCL11 and CCR3 in human dermal fibroblasts, we performed an enzyme-linked immunosorbent assay for CCL11 and a Western blot assay for CCR3. The TNF-alpha-induced expression of CCL11 and CCR3 genes was attenuated by Rosmarinic acid. 3. In our NF-kappaB luciferase reporter system, TNF-alpha-induced NF-kappaB activation was observed to be reduced by Rosmarinic acid. In accordance with this result, Rosmarinic acid also inhibited TNF-alpha-induced phosphorylation and degradation of IkappaB-alpha, as well as nuclear translocation of NF-kappaB heterodimer induced by TNF-alpha. This suggests that Rosmarinic acid downregulates the expression of CCL11 and CCR3 via the inhibition of NF-kappaB activation signaling. 4. Using the NF-kappaB luciferase reporter system, Western blot analysis, and IKK-beta activity assay, we determined that Rosmarinic acid inhibits IKK-beta activity in NF-kappaB signaling, which upregulates the expression of CCL11 and CCR3. Additionally, TNF-alpha-induced secretion of soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 molecules was found to be attenuated by Rosmarinic acid.
CONCLUSIONS:
5. Our results show that Rosmarinic acid inhibits the expression of CCL11 and CCR3 by suppressing the IKK-beta activity in NF-kappaB activation signaling. Further, these results suggest that Rosmarinic acid might inhibit the expression of NF-kappaB promoter-related genes.
2016 Jul 25;254:135-45.
Combining in vitro and in silico approaches to evaluate the multifunctional profile of rosmarinic acid from Blechnum brasiliense on targets related to neurodegeneration[Pubmed:
27270453]
Natural products are important sources of chemical diversity leading to unique scaffolds that can be exploited in the discovery of new drug candidates or chemical probes. In this context, chemical and biological investigation of ferns and lycophytes occurring in Brazil is an approach adopted by our research group aiming at discovering bioactive molecules acting on neurodegeneration targets. In the present study, Rosmarinic acid (RA) isolated from Blechnum brasiliense showed an in vitro multifunctional profile characterized by antioxidant effects, and monoamine oxidases (MAO-A and MAO-B) and catechol-O-methyl transferase (COMT) inhibition. RA showed antioxidant effects against hydroxyl (HO(•)) and nitric oxide (NO) radicals (IC50 of 29.4 and 140 μM, respectively), and inhibition of lipid peroxidation (IC50 of 19.6 μM). In addition, RA inhibited MAO-A, MAO-B and COMT enzymes with IC50 values of 50.1, 184.6 and 26.7 μM, respectively. The MAO-A modulation showed a non-time-dependent profile, suggesting a reversible mechanism of inhibition. Structural insights on RA interactions with MAO-A and COMT were investigated by molecular docking. Finally, RA (up to 5 mM) demonstrated no cytotoxicity on polymorphonuclear rat cells. Taken together, our results suggest that RA may be exploited as a template for the development of new antioxidant molecules possessing additional MAO and COMT inhibition effects to be further investigated on in vitro and in vivo models of neurodegenerative diseases.
Keywords: Antioxidant; Blechnum brasiliense; COMT; MAO-A; Molecular docking; Rosmarinic acid.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
2016 Jan;24(1):75-84.
Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells[Pubmed:
26759705]
This study was designed to investigate the cytoprotective effect of Rosmarinic acid (RA) on ultraviolet B (UVB)-induced oxidative stress in HaCaT keratinocytes. RA exerted a significant cytoprotective effect by scavenging intracellular ROS induced by UVB. RA also attenuated UVB-induced oxidative macromolecular damage, including protein carbonyl content, DNA strand breaks, and the level of 8-isoprostane. Furthermore, RA increased the expression and activity of superoxide dismutase, catalase, heme oxygenase-1, and their transcription factor Nrf2, which are decreased by UVB radiation. Collectively, these data indicate that RA can provide substantial cytoprotection against the adverse effects of UVB radiation by modulating cellular antioxidant systems, and has potential to be developed as a medical agent for ROS-induced skin diseases.
Keywords: Antioxidant system; Oxidative stress; Reactive oxygen species; Rosmarinic acid; Ultraviolet B.
Mol Cell Biochem. 2015 Mar 4.
Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic rats.[Pubmed:
25735949]
Persistent hyperglycemia and elevated levels of free fatty acids (FFA) contribute to oxidative stress, a proximate cause for the onset and progression of diabetes and its complications.
METHODS AND RESULTS:
The present study was hypothesized to evaluate the anti-diabetic potential of Rosmarinic acid (RA) during high-fat diet (HFD)-streptozotocin (STZ)-induced type 2 Diabetes (T2D) in wistar albino rats. Oral administration of RA (100 mg/kg b.w) significantly (p < 0.05) increased the insulin sensitivity index (ISI0,120), while the levels of blood glucose, HbA1c, advanced glycation end products (AGE), TNF-α, IL-1β, IL 6, NO, p-JNK, P38 MAPK and NF-κB were significantly reduced, with a concomitant elevation in the plasma insulin levels in diabetic rats. Furthermore, RA treatment significantly (p < 0.05) reduced the levels of triglycerides, FFA and cholesterol in serum, and reduced the levels of lipid peroxides, AOPP's and protein carbonyls in the plasma and pancreas of diabetic rats. The diminished activities of pancreatic superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) and the decreased levels of plasma ceruloplasmin, vitamin C, vitamin E and reduced glutathione (GSH) in diabetic rats were also significantly (p < 0.05) recovered upon RA treatment denoting its antioxidant potential which was confirmed by Nrf-2, hemeoxyenase (HO-1) levels. Histological, ultrastructural and immunohistochemical data demonstrate that oral administration of RA protects pancreatic β-cells from oxidative niche in HFD-STZ-induced experimental diabetes.
CONCLUSIONS:
Our findings suggest that the oral treatment with RA alleviates pancreatic β-cell dysfunction and glucolipotoxicity-mediated oxidative stress during HFD-STZ-induced T2DM, perhaps through its antioxidant potential.
Biomol Ther (Seoul). 2016 Jan;24(1):75-84.
Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells.[Pubmed:
26759705 ]
Cell lines:HaCaT cells
Concentrations: 0.625, 1.25, 2.5, or 5 μM
Incubation Time: 48 h
Method:
Cells are treated with RA (0.625, 1.25, 2.5, or 5 μM) and exposed to UVB radiation 1 h later. They are then incubated at 37°C for 48 h. At this time, MTT is added to each well to obtain a total reaction volume of 200 μl. After 4 h incubation, the supernatant is removed by aspiration. The formazan crystals in each well are dissolved in dimethyl sulfoxide (DMSO; 150 μl), and the absorbance at 540 nm is measured on a scanning multi-well spectrophotometer.
Life Sci. 2015 Feb 1;122:65-71.
Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice.[Pubmed:
25498895]
Animal Models: Male CF-1 mice
Formulation: ---
Dosages:1, 2 or 4mg/kg, once every three days during 16days
Administration: i.p.