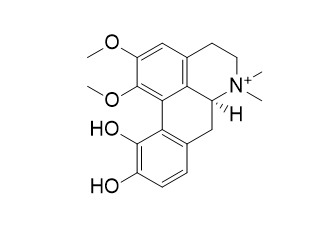
Fuzitine
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2021, 26(4):817.
Appl. Sci.2020, 10(4),1304
Inflammation.2021, doi: 10.1007
Korean Journal of Pharmacognosy2019, 50(4):285-290
Biology (Basel).2020, 9(11):363.
Braz J Biol.2023, 82:e266573.
Nutrients2020, 12(3):811.
Research Square2020, doi: 10.21203.
Mol Med Rep.2024, 29(2):26.
Toxicol Appl Pharmacol.2022, 434:115815.
Related and Featured Products
Chem Pharm Bull (Tokyo) . 2010 Mar;58(3):415-7
New feruloyl tyramine glycosides from Stephania hispidula YAMAMOTO[Pubmed:
20190454]
Three new feruloyl tyramine glycosides, N-cis-feruloyl tyramine-4'''-O-beta-D-glucopyranoside (1), N-trans-ferloyl tyramine-4'''-O-beta-D-glucopyranoside (2), and N-trans-feruloyl tyramine-4'-O-beta-D-glucopyranoside (3), along with six known compounds, N-trans-feruloyl-3'''-methoxydopamine-4'-O-beta-D-glucopyranoside (4), haitinosporine (5), tubocurine (6), Fuzitine (7), (+)-lyoniresinol-3alpha-O-beta-D-glucopyranoside (8), and (-)-lyoniresinol-2alpha-O-beta-D-glucopyranoside (9), were isolated from the stem of Stephania hispidula YAMAMOTO. The structures were elucidated by spectroscopic and chemical analysis.
Yao Xue Xue Bao . 1992;27(9):670-3.
Studies on the alkaloid constituents of Jiangyou fu-zi Aconitum carmichaeli from Sichuan[Pubmed:
1293938]
Six compounds were isolated from the aqueous extract of Aconitum carmichaeli Debx (cultivated in Jiang-you region of Sichuan province). Five of them have been identified as uracil (I), songorine HCl(II), karakoline(III), neoline(IV), and Fuzitine(VI). Compound V is a new C19-diterpenoid alkaloid determined as C33H47NO9 and named neojiangyouaconitine.
Yao Xue Xue Bao . 1992;27(9):670-673.
[Studies on the alkaloid constituents of Jiangyou fu-zi Aconitum carmichaeli from Sichuan][Pubmed:
1293938]
Six compounds were isolated from the aqueous extract of Aconitum carmichaeli Debx (cultivated in Jiang-you region of Sichuan province). Five of them have been identified as uracil (I), songorine HCl(II), karakoline(III), neoline(IV), and Fuzitine(VI). Compound V is a new C19-diterpenoid alkaloid determined as C33H47NO9 and named neojiangyouaconitine.
Chem Pharm Bull (Tokyo) . 2010 Mar;58(3):415-417.
New feruloyl tyramine glycosides from Stephania hispidula YAMAMOTO[Pubmed:
20190454]
Three new feruloyl tyramine glycosides, N-cis-feruloyl tyramine-4'''-O-beta-D-glucopyranoside (1), N-trans-ferloyl tyramine-4'''-O-beta-D-glucopyranoside (2), and N-trans-feruloyl tyramine-4'-O-beta-D-glucopyranoside (3), along with six known compounds, N-trans-feruloyl-3'''-methoxydopamine-4'-O-beta-D-glucopyranoside (4), haitinosporine (5), tubocurine (6), Fuzitine (7), (+)-lyoniresinol-3alpha-O-beta-D-glucopyranoside (8), and (-)-lyoniresinol-2alpha-O-beta-D-glucopyranoside (9), were isolated from the stem of Stephania hispidula YAMAMOTO. The structures were elucidated by spectroscopic and chemical analysis.
Chem Pharm Bull (Tokyo) . 2004 Apr;52(4):454-455.
A new alkaloid and its artificial derivative with an indazole ring from Nigella glandulifera[Pubmed:
15056964]
A new compound, nigeglanine (1), and its new artificial derivative (1a), were isolated from the seeds of Nigella glandulifera, together with a known aporphine alkaloid, Fuzitine (2). Their structures were established by spectral analysis, including two-dimensional (2D)-NMR spectroscopy. Nigeglanine (1) is the third natural product determined to contain an indazole nucleus.