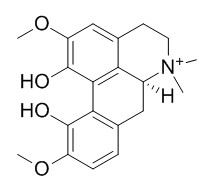
Magnoflorine
Magnoflorine possesses high activity as α-glucosidase inhibitor in vitro and in vivo, has antidiabetic potential activity; it also has sedative and anxiolytic effects, probably mediated by a GABAergic mechanism of action. Magnoflorine has protective effects, mediated by some mechanism other than prevention of micelle formation or protection of the erythrocyte membrane against osmotic imbalance.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2021, 93:153789.
Chulalongkorn University2024, ssrn.4716057.
J Sep Sci.2020, 43(22):4148-4161.
Industrial Crops and Products2018, 353-362
Current Analytical Chemistry2024, 20(8):599-610.
Evid Based Complement Alternat Med.2016, 2016:4357656
Heliyon.2024, 10(12):e31722.
Int J Mol Sci.2018, 19(9):E2528
Phytochemistry.2021, 181:112539.
Pharmaceutics.2020, 12(9):845.
Related and Featured Products
J Nat Med. 2015 Apr 4.
Sinomenine and magnoflorine, major constituents of Sinomeni Caulis et Rhizoma, show potent protective effects against membrane damage induced by lysophosphatidylcholine in rat erythrocytes.[Pubmed:
25840917 ]
The effects of the water extract of Sinomeni Caulis et Rhizoma (SCR-WE) and its major constituents, sinomenine (SIN) and Magnoflorine (MAG), on moderate hemolysis induced by lysophosphatidylcholine (LPC) were investigated in rat erythrocytes and compared with the anti-hemolytic effects of lidocaine (LID) and propranolol (PRO) as reference drugs.
METHODS AND RESULTS:
LPC caused hemolysis at concentrations above the critical micelle concentration (CMC), and the concentration of LPC producing moderate hemolysis (60 %) was approximately 10 μM. SCR-WE at 1 ng/mL-100 μg/mL significantly inhibited the hemolysis induced by LPC. SIN and Magnoflorine attenuated LPC-induced hemolysis in a concentration-dependent manner from very low to high concentrations (1 nM-100 μM and 10 nM-100 μM, respectively). In contrast, the inhibiting effects of LID and PRO on LPC-induced hemolysis were observed at higher concentrations (1-100 μM) but not at lower concentrations (1-100 nM). Neither SIN nor Magnoflorine affected micelle formation of LPC, nor, at concentrations of 1 nM-1 μM, did they attenuate the hemolysis induced by osmotic imbalance (hypotonic hemolysis). Similarly, SCR-WE also did not modify micelle formation or hypotonic hemolysis, except at the highest concentration.
CONCLUSIONS:
These results suggest that SIN and Magnoflorine potently protect the erythrocyte membrane from LPC-induced damage and contribute to the beneficial action of SCR-WE. The protective effects of SIN and Magnoflorine are mediated by some mechanism other than prevention of micelle formation or protection of the erythrocyte membrane against osmotic imbalance.
Biol Pharm Bull. 2007 Jun;30(6):1157-60.
Magnoflorine from Coptidis Rhizoma protects high density lipoprotein during oxidant stress.[Pubmed:
17541173]
The objective of the present study was to investigate the beneficial properties of Magnoflorine, an alkaloid isolated from coptidis rhizoma, on protecting human high density lipoprotein (HDL) against lipid peroxidation.
METHODS AND RESULTS:
Magnoflorine exerts an inhibitory effect against Cu2+-induced lipid peroxidation of HDL, as showed by prolongation of lag time from 62 to 123 min at the concentration of 3.0 microM. It also inhibits the generation of thiobarbituric acid reactive substances (TBARS) in the dose-dependent manners with IC50 values of 2.3+/-0.2 microM and 6.2+/-0.5 microM since HDL oxidation mediated by either catalytic Cu2+ or thermo-labile radical initiator (AAPH), respectively. Separately, Cu2+ oxidized HDL lost the antioxidant action but the inclusion of Magnoflorine/Cu2+ oxidized HDL can protect LDL oxidation according to increasing Magnoflorine concentration.
CONCLUSIONS:
The results suggest that Magnoflorine may have a role to play in preventing the HDL oxidation.
World J Microbiol Biotechnol . 2018 Oct 31;34(11):167.
Antifungal activity of magnoflorine against Candida strains[Pubmed:
30382403]
Abstract
Candida albicans is a major invasive pathogen, and the development of strains resistant to conventional antifungal agents has been reported in recent years. We evaluated the antifungal activity of 44 compounds against Candida strains. Magnoflorine showed the highest growth inhibitory activity of the tested Candida strains, with a minimum inhibitory concentration (MIC) of 50 μg/mL based on microdilution antifungal susceptibility testing. Disk diffusion assay confirmed the antifungal activity of Magnoflorine and revealed that this activity was stable over 3 days compared to those of berberine and cinnamaldehyde. Cytotoxicity testing showed that Magnoflorine could potentially be used in a clinical setting because it didn't have any toxicity to HaCaT cells even in 200 μg/mL of treatment. Magnoflorine at 50 μg/mL inhibited 55.91 ± 7.17% of alpha-glucosidase activity which is required for normal cell wall composition and virulence of Candida albicans. Magnoflorine also reduced the formation of C. albicans' biofilm. Combined treatment with Magnoflorine and miconazole decreased the amount of miconazole required to kill various Candida albicans. Therefore, Magnoflorine is a good candidate lead compound for novel antifungal agents.
Keywords: Alpha-glucosidase; Antifungal; Candida albicans; Magnoflorine; Susceptibility microdilution assay.
Planta Med . 2018 Nov;84(17):1255-1264.
Magnoflorine Enhances LPS-Activated Pro-Inflammatory Responses via MyD88-Dependent Pathways in U937 Macrophages[Pubmed:
29906814]
Abstract
Magnoflorine, a major bioactive metabolite isolated from Tinospora crispa, has been reported for its diverse biochemical and pharmacological properties. However, there is little report on its underlying mechanisms of action on immune responses, particularly on macrophage activation. In this study, we aimed to investigate the effects of Magnoflorine, isolated from T. crispa on the pro-inflammatory mediators generation induced by LPS and the concomitant NF-κB, MAPKs, and PI3K-Akt signaling pathways in U937 macrophages. Differentiated U937 macrophages were treated with Magnoflorine and the release of pro-inflammatory mediators was evaluated through ELISA, while the relative mRNA expression of the respective mediators was quantified through qRT-PCR. Correspondingly, western blotting was executed to observe the modulatory effects of Magnoflorine on the expression of various markers related to NF-κB, MAPK and PI3K-Akt signaling activation in LPS-primed U937 macrophages. Magnoflorine significantly enhanced the upregulation of TNF-α, IL-1β, and PGE2 production as well as COX-2 protein expression. Successively, Magnoflorine prompted the mRNA transcription level of these pro-inflammatory mediators. Magnoflorine enhanced the NF-κB activation by prompting p65, IκBα, and IKKα/β phosphorylation as well as IκBα degradation. Besides, Magnoflorine treatments concentration-dependently augmented the phosphorylation of JNK, ERK, and p38 MAPKs as well as Akt. The immunoaugmenting effects were further confirmed by investigating the effects of Magnoflorine on specific inhibitors, where the treatment with specific inhibitors of NF-κB, MAPKs, and PI3K-Akt proficiently blocked the Magnoflorine-triggered TNF-α release and COX-2 expression. Magnoflorine furthermore enhanced the MyD88 and TLR4 upregulation. The results suggest that Magnoflorine has high potential on augmenting immune responses.
J. Funct. Foods, 2012, 4(1):79-86.
Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and is antiglycemic in rats.[Reference:
WebLink]
Antidiabetic potential of Tinospora cordifolia stem is well proven. In the course of screening of useful α-glucosidase inhibitors, we prepared alkaloid fraction (AFTC) and isolated three isoquinoline alkaloids, namely, jatrorrhizine, palmatine and Magnoflorine as active candidates for α-glucosidase inhibition.
METHODS AND RESULTS:
The enzyme kinetics was studied using sucrose and maltose as substrates. Michaelis–Menten constant (Km) and maximal velocity (Vmax) values were estimated. A significant decrease in Vmax and unaltered Km was observed in case of jatrorrhizine and palmatine (non-competitive inhibition). Magnoflorine was found to increase apparent Km and shown to be reversible, competitive inhibition. The IC50 value as sucrase inhibitor was 36.25, 23.46 and 9.8μg/mL for jatrorrhizine, palmatine and Magnoflorine, respectively, and as maltase inhibitor was 22.05, 38.42 and 7.6μg/mL for jatrorrhizine, palmatine and Magnoflorine, respectively. In vivo studies were conducted on rats to determine oral glucose tolerance test (OGTT), using different substrates: glucose, sucrose and maltose. The increase in plasma glucose level was significantly suppressed (P<0.01) by all the three alkaloids at 20mg/kg b.w.
CONCLUSIONS:
Magnoflorine possessed the most potential activity as α-glucosidase inhibitor in vitro and in vivo.
J Nat Med. 2013 Oct;67(4):814-21.
The involvement of magnoflorine in the sedative and anxiolytic effects of Sinomeni Caulis et Rhizoma in mice.[Pubmed:
23456265]
The present study seeks to evaluate the sedative and anxiolytic effects of the 70% ethanol extract of Sinomeni Caulis et Rhizoma (SR).
METHODS AND RESULTS:
The extract was orally administered to mice at dosages of 25, 50, 100, 200 or 400 mg/kg. The mice were then subjected to an array of behavioral tests to assess the sedative (open-field, rota-rod, and thiopental sodium-induced sleeping test) and anxiolytic (elevated plus maze test) effects of the substance. SR (100, 200 mg/kg) significantly reduced locomotor activity, decreased rota-rod performance, and potentiated thiopental sodium-induced sleeping in mice, all indicative of its sedative effects. SR (50, 100 mg/kg) also produced anxiolytic effects, as shown by an increase in entries and staying time on the open arm of the plus maze. SR's sedative and anxiolytic effects were comparable to that of the benzodiazepine, diazepam. Moreover, to identify SR's probable mechanism of action, intracellular Cl⁻ ion influx was observed in cultured human neuroblastoma cells. SR dose-dependently increased Cl⁻ influx, which was blocked by co-administration of the GABAA receptor competitive antagonist, bicuculline. Among the major constituents of SR, only Magnoflorine showed a similar increment in Cl⁻ influx, which was also blocked by bicuculline.
CONCLUSIONS:
Altogether, the present results suggest that SR has sedative and anxiolytic effects, probably mediated by Magnoflorine through a GABAergic mechanism of action.