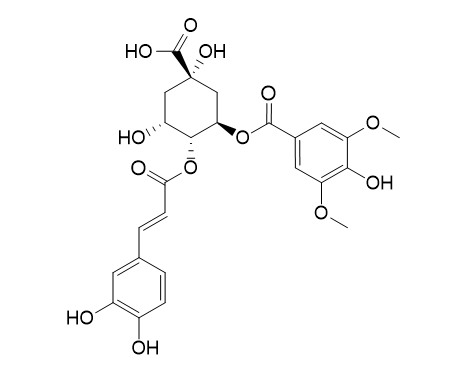
4-O-Caffeoyl-3-O-syringoylquinic acid
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2017, 22(11)
Metabolites2023, 13(1), 3.
Molecules.2022, 27(21):7643.
Molecules.2019, 24(9):E1719
PLoS One.2022, 17(6):e0268505.
Anal Bioanal Chem. 2025, 417(17):3879-3892.
Molecules.2021, 26(2):313.
Molecules.2022, 27(19):6651.
Agronomy2023, 13(9), 2410.
Ann Transl Med.2019, 7(23):731
Related and Featured Products
Planta Med . 2013 Nov;79(16):1558-1564.
Chemical constituents from the roots and stems of Erycibe obtusifolia and their in vitro antiviral activity[Pubmed:
24081686]
Three new quinic acid derivatives, 4-O-caffeoyl-3-O-sinapoylquinic acid methyl ester (1), 5-O-caffeoyl-4-O-syringoylquinic acid methyl ester (2), and 4-O-Caffeoyl-3-O-syringoylquinic acid methyl ester (3), as well as four new coumarin glycosides, 7-O-(3-O-sinapoyl-β-D-glucopyranosyl)-6-methoxycoumarin (12), 7-O-(6-O-sinapoyl-β-D-glucopyranosyl)-6-methoxycoumarin (13), 7-O-(2-O-sinapoyl-β-D-glucopyranosyl)-6-methoxycoumarin (14), and 7-O-(6-O-syringoyl-β-D-glucopyranosyl)-6-methoxycoumarin (15), together with eight known compounds (4-11) were isolated from the roots and stems of Erycibe obtusifolia. Their structures were elucidated on the basis of spectroscopic analysis and chemical evidence. All the compounds were screened for their in vitro antiviral activity against respiratory syncytial virus with a cytopathic effect reduction assay. Among them, the di-O-caffeoyl quinates 8-11 displayed a potent in vitro anti-respiratory syncytial virus effect.
Fitoterapia . 2014 Dec;99:109-116.
Acyl quinic acid derivatives from the stems of Erycibe obtusifolia[Pubmed:
25256062]
Eleven new acyl quinic acid derivatives, 4-O-Caffeoyl-3-O-syringoylquinic acid methyl ester (1), 4-O-caffeoyl-3-O-vanilloylquinic acid (2), 4-O-caffeoyl-3-O-vanilloylquinic acid methyl ester (3), 5-O-caffeoyl-3-O-vanilloylquinic acid (4), 5-O-caffeoyl-3-O-vanilloylquinic acid methyl ester (5), 5-O-caffeoyl-3-O-sinapoylquinic acid (6), 5-O-caffeoyl-4-O-vanilloylquinic acid (7), 4-O-(7‴S, 8‴R)-glycosmisoyl-5-O-caffeoylquinic acid methyl ester (8), 4-O-(7‴S, 8‴R)-glycosmisoyl-5-O-caffeoylquinic acid (9), 3-O-(7‴S, 8‴R)-glycosmisoyl-4-O-caffeoylquinic acid (10), and 3-O-(7‴S, 8‴R)-glycosmisoyl-4-O-caffeoylquinic acid methyl ester (11), have been isolated from the stems of Erycibe obtusifolia together with eight known compounds (12-19). Their structures were elucidated on the basis of spectroscopic data analysis (UV, IR, HRESIMS, CD, and 1D and 2D NMR) and chemical evidence. In in vitro assay, compounds 7 and 16-18 exhibited significant neuroprotective effects against rotenone induced PC12 cell damage at 10 μM.
3''-Galloylquercitrin
Catalog No: CFN95048
CAS No: 503446-90-0
Price: $368/5mg
Glyasperin C
Catalog No: CFN95065
CAS No: 142474-53-1
Price: $333/10mg
Scutellarein-7-O-glucoside
Catalog No: CFN95082
CAS No: 26046-94-6
Price: $318/10mg
Caraganaphenol A
Catalog No: CFN95093
CAS No: 174916-31-5
Price: $333/5mg
Rhamnocitrin 3-glucoside
Catalog No: CFN95134
CAS No: 41545-37-3
Price: $318/10mg
1,7-Diphenyl-5-hydroxy-4,6-hepten-3-one
Catalog No: CFN95171
CAS No: 87095-77-0
Price: $318/10mg
Bidenoside C
Catalog No: CFN95266
CAS No: 700877-55-0
Price: $318/5mg
1,3,4,6-Tetragalloylglucose
Catalog No: CFN95425
CAS No: 26922-99-6
Price: $318/10mg
trans-Cinnamoyl beta-D-glucoside
Catalog No: CFN95497
CAS No: 13080-39-2
Price: Inquiry(manager@chemfaces.com)
12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No: CFN95505
CAS No: 1309931-91-6
Price: $318/5mg