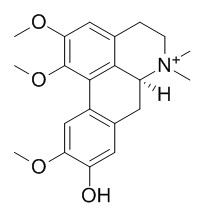
Xanthoplanine
Xanthoplanine fully inhibits the EC(50) ACh responses of both alpha7 and alpha4beta2 nACh receptors with estimated IC(50) values of 9+/-3 microM (alpha7) and 5+/-0.8 microM (alpha4beta2).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Immunol.2017, 8:1542
Int J Biol Macromol.2020, 169:342-351
Pharmacia2024, 71:1-9.
Molecules.2019, 24(22):E4022
Agronomy2023, 13(9), 2410.
Food Bioscience2023, 53:102687
Int J Nanomedicine.2022, 17:6513-6525.
Biomed Chromatogr.2022, 36(11):e5462.
J Cell Mol Med.2023, jcmm.17968.
Int J Mol Sci.2023, 24(18):14077.
Related and Featured Products
Bioorg Med Chem. 2007 May 15;15(10):3368-72.
Aporphine metho salts as neuronal nicotinic acetylcholine receptor blockers.[Pubmed:
17391965]
METHODS AND RESULTS:
(S)-Aporphine metho salts with the 1,2,9,10 oxygenation pattern displaced radioligands from recombinant human alpha7 and alpha4beta2 neuronal nicotinic acetylcholine receptors (nAChR) at low micromolar concentrations. The affinity of the nonphenolic glaucine methiodide (4) (vs [(3)H]cytisine) was the lowest at alpha4beta2 nAChR (K(i)=10 microM), and predicentrine methiodide (2) and Xanthoplanine iodide (3), with free hydroxyl groups at C-2 or C-9, respectively, had the highest affinity at these receptors (K(i) approximately 1 microM), while the affinity of the diphenolic boldine methiodide (1) was intermediate between these values. At homomeric alpha7 nAChR, Xanthoplanine had the highest affinity (K(i)=10 microM) vs [(125)I]alpha-bungarotoxin while the other three compounds displaced the radioligand with K(i) values between 15 and 21 microM. At 100 microM, all four compounds inhibited the responses of these receptors to EC(50) concentrations of ACh.
CONCLUSIONS:
The effects of Xanthoplanine iodide (3) were studied in more detail. Xanthoplanine fully inhibited the EC(50) ACh responses of both alpha7 and alpha4beta2 nACh receptors with estimated IC(50) values of 9+/-3 microM (alpha7) and 5+/-0.8 microM (alpha4beta2).
Phytochemistry. 2004 Apr;65(7):939-44.
Quaternary isoquinoline alkaloids from Xylopia parviflora.[Pubmed:
15081299]
METHODS AND RESULTS:
From the quaternary alkaloidal fraction of the bark and the root of Xylopia parviflora (Annonaceae), four isoquinoline alkaloids, xylopinidine, dehydrocoreximine, N, N-dimethylanomurine and N-methylphoebine were isolated along with the known compounds, pycnarrhine, lotusine, 6,7-dimethoxy-2-methyl-isoquinolinium salt, 1,2-dehydroreticuline, (-)-phellodendrine, (+)-tembetarine, (-)-litcubine, (+)-magnoflorine, tetradehydroreticuline, (-)-oblongine, (+)-menisperine, (+)-N-methylcorydine, stepharanine, (+)-Xanthoplanine, dehydrodiscretine, jatrorrhizine and palmatine.
CONCLUSIONS:
3,4-Dihydro-6,7-dimethoxy-2-methyl-isoquinolinium and N-methylpurpuerine were isolated as natural products for the first time. Their structures were determined on the basis of spectroscopic evidence.