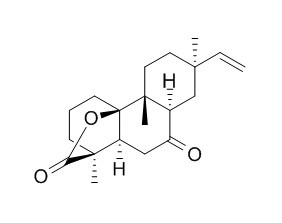
Rosenonolactone
Rosenonolactone shows inhibitory activity against prolyl endopeptidase and thrombin.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chinese J of Tissue Engineering Res.2022, 26(17): 2636-2641.
Int J Mol Sci.2017, 18(12)
Chem Biol Interact.2024, 394:110995.
J Agric Food Chem.2020, 68(51):15164-15175
Int J Mol Sci.2024, 25(19):10660.
Pharmacognosy Journal.2020, 12(2), p232-235.
J Ethnopharmacol.2020, 269:113752.
Sains Malaysiana2022, 51(4):1143-1154
Molecules.2023, 28(9):3685.
Molecules.2019, 24(2):329
Related and Featured Products
Mycotoxin Res. 1992 Sep;8(2):77-83.
DNA strand break induction, mutagenicity, and cytotoxicity of the mycotoxins 11-β-hydroxy-7-deoxy-rosenonolactone, rosenonolactone, and trichothecin.[Pubmed:
23606003]
11-β-hydroxy-7-deoxy-Rosenonolactone (TSS1), a mycotoxin of the rosenane class, was tested on cytotoxicity, induction of DNA single strand breaks and muta-genicity. Its effects were compared to those of Rosenonolactone and trichothecin.
METHODS AND RESULTS:
TSS1 had stronger antibiotic activity againstEscherichia coli (EC 50: 10μg/mL) than Rosenonolactone (EC 50: >200μg/mL) but weaker activity than trichothecin (EC 50: 3μg/mL). The same order of activity was found for the inhibition of yeast fermentation (EC 50 of TSS1: 45μg/mL; EC 50 of Rosenonolactone: > 120μg/mL; EC 50 of trichothecin: 3.4μg/mL).In the trypan blue exclusion test using V79 Chinese hamster cells, TSS1 proved to be cytotoxic (EC50: 30μg/mL) at even lower doses than trichothecin (EC50: 200μg/mL). Rosenonolactone had no significant toxicity up to the highest soluble concentration (500μg/mL).DNA single strand breaks caused by TSS1 occurred at the same concentrations at which damage of the cell membrane became apparent. For trichothecin single strand breaks were detected only at concentrations at which the membrane was already highly damaged. No single strand breaks were observed in V79 cells after incubation with Rosenonolactone up to the limit of solubility (500μg/mL).
CONCLUSIONS:
In the reversion assay withhis Salmonella typhimurium strains TA 98 and TA 100, no mutagenicity was observed for any of the examined mycotoxins up to 800μg/plate with and without the addition of a rat liver preparation for metabolism of the test compound.
Chem Pharm Bull (Tokyo). 2002 Apr;50(4):515-8.
Enzyme inhibitory constituents from Duranta repens.[Pubmed:
11964000]
METHODS AND RESULTS:
Isoprenylated flavonoids 5,7-dihydroxy-3'-(2-hydroxy-3-methyl-3-butenyl)-3,6,4'-trimethoxyflavone (1), 3,7-dihydroxy-3'-(2-hydroxy-3-methyl-3-butenyl)-5,6,4'-trimethoxyflavone (2) and an isoprenylated acetophenone derivative (3) have been isolated from Duranta repens along with known compounds, 5-hydroxy-3,6,7,4'-tetramethoxyflavone (4), Rosenonolactone (5), 6,7-dimethoxycoumarin (6), 5alpha,8alpha-epidioxyergosta-6,22-dien-3beta-ol (7) and 5alpha,8alpha-epidioxyergosta-6,9(11),22-trien-3beta-ol (8), isolated for the first time from this species. Their structures and the relative configuration were determined by spectroscopic methods (1H- and 13C-NMR, IR, UV and MS) and two-dimensional (2D)-NMR experiments.
CONCLUSIONS:
The compounds 1-5 showed inhibitory activity against prolyl endopeptidase while 4 and 5 were also active against thrombin.