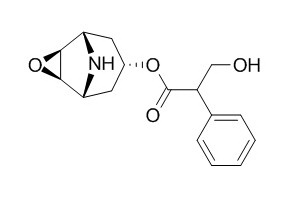
Norscopolamine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules. 2013, 18(11):14105-21
mBio.2020, 11(3):e00686-20.
Applied Biological Chemistry2020, 63:33(2020)
J. Traditional Thai Medical Res. 2022,8(1):1-14.
Pharmaceutical Chemistry Journal2019, 52(12):986-991
J Food Sci.2021, 86(9):3810-3823.
Tissue Cell.2024, 88:102401.
Molecules.2019, 24(6):E1177
Biocell2023, 47(8):1793-1802
Naunyn Schmiedebergs Arch Pharmacol.2024, 03148-x.
Related and Featured Products
Green Chemistry, 2012, 14(4):1189-1195.
One-pot oxidative N-demethylation of tropane alkaloids with hydrogen peroxide and a FeIII-TAML catalyst.[Reference:
WebLink]
METHODS AND RESULTS:
The oxidative N-demethylation of tropane alkaloids to their nortropane derivatives has been investigated using H2O2 and an iron(III) tetraamido macrocycle (FeIII-TAML) catalyst. The yields of the nortropanes were found to be dependent on the amount of H2O2 used in the reaction, the catalyst loading, the nature of the organic co-solvent and the type of tropine substrate. N-Hydroxy-nortropane, N-formyl-nortropane and tropane-N-oxide derivatives were identified as by-products of the reaction.
CONCLUSIONS:
After screening various reaction conditions, the optimised conditions were applied to the N-demethylation of atropine and scopolamine at preparative scales and the desired products, noratropine and Norscopolamine, obtained following one pot reactions in good yields and high purity without the need for any chromatographic purification steps.