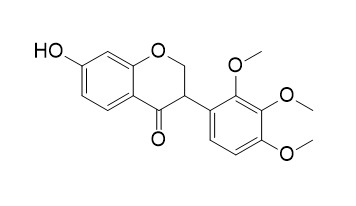
3'-O-Methylviolanone
3'-O-methylviolanone has anti-inflammatory activity .
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Sci Food Agric.2018, 98(3):1153-1161
Int J Mol Sci.2024, 25(18):10219.
bioRxiv2021, 462065.
Current Analytical Chemistry2024, 20(8):599-610.
Kangwon National University2022, 37(1):29-37
J Ethnopharmacol.2024, 326:117902.
FEBS J.2022, 10.1111:febs.16676.
Molecules.2019, 24(6):E1177
PLoS One.2022, 17(6):e0268505.
Phytochem Anal.2021, 32(6):970-981.
Related and Featured Products
Planta Med . 1998 Mar;64(2):153-8. doi: 10.1055/s-2006-957394.
Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera[Pubmed:
9525107]
Three new flavonoids, (3R)-4'-methoxy-2',3,7-trihydroxyisoflavanone (11), 7-methoxy-3,3',4',6-tetrahydroxyflavone (18), and 2',7-dihydroxy-4',5'-dimethoxyisoflavone (22), were isolated from the heartwood of Dalbergia odorifera T. Chen. (Leguminosae), together with twenty-two known compounds, (S)-4-methoxydalbergione (1), cearoin (2), medicarpin (3), formononetin (4), sativanone (5), 3-hydroxy-9-methoxy-coumestan (6), meliotocarpan A (7), isoliquiritigenin (8), stevein (9), liquiritigenin (10), 3',4',7-trihydroxyflavanone (12), butein (13), 3'-hydroxymelanettin (14), koparin (15), bowdichione (16), fisetin (17), melanettin (19), sulfuretin (20), 3'-hydroxydaidzein (21), 3'-O-Methylviolanone (23), xenognosin B (24), and dalbergin (25). These flavonoids were evaluated in antiallergic and anti-inflammatory tests. The results showed that (S)-4-methoxydalbergione (1) and cearoin (2) exhibited antiallergic activity while (S)-4-methoxydalbergione (1), cearoin (2), butein (13), koparin (15), bowdichione (16), 3'-O-Methylviolanone (23), and xenognosin B (24) showed significant anti-inflammatory activity.
Molecules . 2021 Sep 4;26(17):5381.
Dihydroisocoumarins and Dihydroisoflavones from the Rhizomes of Dioscorea collettii with Cytotoxic Activity and Structural Revision of 2,2'-Oxybis(1,4-di-tert-butylbenzene)[Pubmed:
34500814]
The investigation of the constituents of the rhizomes of Dioscorea collettii afforded one new dihydroisocoumarin, named (-)-montroumarin (1a), along with five known compounds-montroumarin (1b), 1,1'-oxybis(2,4-di-tert-butylbenzene) (2), (3R)-3'-O-Methylviolanone (3a), (3S)-3'-O-Methylviolanone (3b), and (RS)-sativanone (4). Their structures were elucidated using extensive spectroscopic methods. To the best of our knowledge, compound 1a is a new enantiomer of compound 1b. The NMR data of compound 2 had been reported but its structure was erroneous. The structure of compound 2 was revised on the basis of a reinterpretation of its NMR data (1D and 2D) and the assignment of the 1H and 13C NMR data was given rightly for the first time. Compounds 3a-4, three dihydroisoflavones, were reported from the Dioscoreaceae family for the first time. The cytotoxic activities of all the compounds were tested against the NCI-H460 cell line. Two dihydroisocoumarins, compounds 1a and 1b, displayed moderate cytotoxic activities, while the other compounds showed no cytotoxicity.
Antioxidants (Basel) . 2020 Apr 10;9(4):306.
Polyphenols and Sesquiterpene Lactones from Artichoke Heads: Modulation of Starch Digestion, Gut Bioaccessibility, and Bioavailability following In Vitro Digestion and Large Intestine Fermentation[Pubmed:
32290151]
Artichoke is a relevant source of health-promoting compounds such as polyphenols and sesquiterpene lactones. In this study, the bioaccessibility and gut bioavailability of artichoke constituents were evaluated by combining in vitro digestion and large intestine fermentation, metabolomics, and Caco-2 human intestinal cells model. Moreover, the ability of artichoke polyphenols to modulate the in vitro starch digestibility was also explored. An untargeted metabolomic approach based on liquid chromatography quadrupole-time-of-flight (UHPLC/QTOF) mass spectrometry coupled with multivariate statistics was used to comprehensively screen the phytochemical composition of raw, digested, and fermented artichoke. Overall, a large abundance of phenolic acids and sesquiterpene lactones was detected, being 13.77 and 11.99 mg·g-1, respectively. After 20 h of in vitro large intestine fermentation, a decrease in polyphenols and sesquiterpene lactones content was observed. The most abundant compounds characterizing the raw material (i.e., chlorogenic acid and cynaropicrin equivalents) showed an average % bioaccessibility of 1.6%. The highest % bioaccessibility values were recorded for flavonoids such as anthocyanin and flavone equivalents (on average, 13.6%). However, the relatively high bioavailability values recorded for flavonols, phenolic acids, and sesquiterpene lactones (from 71.6% up to 82.4%) demonstrated that these compounds are able to be transported through the Caco-2 monolayer. The phenolic compounds having the highest permeation rates through the Caco-2 model included low molecular weight phenolics such as tyrosol and 4-ethylcatechol; the isoflavonoids 3'-O-Methylviolanone, equol 4'-O-glucuronide, and hydroxyisoflavone; together with the methyl and acetyl derivatives of glycosylated anthocyanins. Therefore, although human in vivo confirmatory trials are deemed possible, current findings provide insights into the mechanistic effects underlying artichoke polyphenols and sesquiterpenoids bioavailability following gastrointestinal and large intestine processes.
Molecules . 2020 Jan 17;25(2):389.
Analysis of Flavonoids in Dalbergia odorifera by Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry[Pubmed:
31963485]
Dalbergia odorifera, a traditional Chinese medicine, has been used to treat cardio- and cerebrovascular diseases in China for thousands of years. Flavonoids are major active compounds in D. odorifera. In this paper, a rapid and sensitive ultra-high performance liquid chromatography-triple quadrupole mass spectrometry method was developed and validated for simultaneous determination of 17 flavonoids in D. odorifera. Quantification was performed by multiple reaction monitoring using electrospray ionization in negative ion mode. Under the optimum conditions, calibration curves for the 17 analytes displayed good linearity (r2 > 0.9980). The intra- and inter-day precisions (relative standard deviations) were lower than 5.0%. The limit of quantitation ranged from 0.256 to 18.840 ng/mL. The mean recovery range at three spiked concentrations was 94.18-101.97%. The validated approach was successfully applied to 18 samples of D. odorifera. Large variation was observed for the contents of the 17 analytes. Sativanone and 3'-O-Methylviolanone were the dominant compounds. The fragmentation behaviors of six flavonoids were investigated using UPLC with quadrupole time-of-flight tandem mass spectrometry. In negative ion electrospray ionization mass spectrometry, all the flavonoids yielded prominent [M - H] ions. Fragments for losses of CH-3, CO, and CO2 were observed in the mass spectra. Formononetin, liquiritigenin, isoliquiritigenin, sativanone, and alpinetin underwent retro-Diels-Alder fragmentations. The proposed method will be helpful for quality control of D. odorifera.
Molecules . 2021 Sep 4;26(17):5381.
Dihydroisocoumarins and Dihydroisoflavones from the Rhizomes of Dioscorea collettii with Cytotoxic Activity and Structural Revision of 2,2'-Oxybis(1,4-di-tert-butylbenzene)[Pubmed:
34500814]
The investigation of the constituents of the rhizomes of Dioscorea collettii afforded one new dihydroisocoumarin, named (-)-montroumarin (1a), along with five known compounds-montroumarin (1b), 1,1'-oxybis(2,4-di-tert-butylbenzene) (2), (3R)-3'-O-Methylviolanone (3a), (3S)-3'-O-Methylviolanone (3b), and (RS)-sativanone (4). Their structures were elucidated using extensive spectroscopic methods. To the best of our knowledge, compound 1a is a new enantiomer of compound 1b. The NMR data of compound 2 had been reported but its structure was erroneous. The structure of compound 2 was revised on the basis of a reinterpretation of its NMR data (1D and 2D) and the assignment of the 1H and 13C NMR data was given rightly for the first time. Compounds 3a-4, three dihydroisoflavones, were reported from the Dioscoreaceae family for the first time. The cytotoxic activities of all the compounds were tested against the NCI-H460 cell line. Two dihydroisocoumarins, compounds 1a and 1b, displayed moderate cytotoxic activities, while the other compounds showed no cytotoxicity.
1,3,6-Tri-O-galloylglucose
Catalog No: CFN95043
CAS No: 18483-17-5
Price: $318/10mg
1-O-galloyl-6-O-cinnamoylglucose
Catalog No: CFN95053
CAS No: 115746-69-5
Price: $338/5mg
3-Acetyl-ginsenoside F1
Catalog No: CFN95238
CAS No: 1881225-08-6
Price: $318/5mg
Lappaol B
Catalog No: CFN95242
CAS No: 62359-60-8
Price: $333/10mg
N1,N5,N10-(E)-tri-p-coumaroylspermidine
Catalog No: CFN95256
CAS No: 364368-18-3
Price: $388/10mg
7-O-(4-beta-D-glucopyranosyloxy-3-methoxybenzoyl)secologanolic acid
Catalog No: CFN95365
CAS No: 469899-55-6
Price: $368/5mg
4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No: CFN95407
CAS No: 85644-03-7
Price: $318/5mg
Bergamjuicin
Catalog No: CFN95422
CAS No: 2376307-20-7
Price: $318/5mg
11beta,13-Dihydrolactucin
Catalog No: CFN95433
CAS No: 83117-63-9
Price: $318/10mg
Methyl nomilinate
Catalog No: CFN95576
CAS No: 77887-51-5
Price: $318/5mg