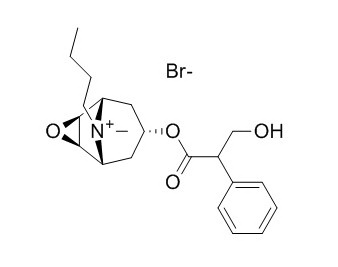
Scopolamine butylbromide
Scopolamine butylbromide is a competitive antagonist of muscarinic acetylcholine receptor (mAChR) with an IC50 of 55.3 ± 4.3 nM, it possesses anticholinergic, and anti-tumor effects, it used as an abdominal-specific antispasmodic agent. Scopolamine butylbromide is effective in preventing succinylcholine-induced bradycardia in infants and children.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Food Drug Anal.2023, 31(2):254-277.
Front Plant Sci.2017, 8:723
Nutrients.2023, 15(4):950.
Anticancer Res.2022, 42(9):4403-4410.
Biol Pharm Bull.2017, 40(6):797-806
J. Food Composition and Anal.2022, V 109:104482.
Vietnam J. Chemistry2022, 60(2):211-222
Processes2021, 9(5),831.
Plants (Basel).2023, 12(11):2107.
Univerzita Karlova2022, 173245.
Related and Featured Products
Clin Psychopharmacol Neurosci. 2015 Apr 30;13(1):109-12.
Effect of Scopolamine Butylbromide on Clozapine-induced Hypersalivation in Schizophrenic Patients: A Case Series.[Pubmed:
25912544]
This study investigated the efficacy of the anticholinergic agent Scopolamine butylbromide against clozapine-induced hypersalivation.
METHODS AND RESULTS:
Five schizophrenia patients were coadministered Scopolamine butylbromide (30-60 mg/ day) for 4 weeks. At the baseline and after 4 weeks' treatment, we subjectively evaluated hypersalivation using a visual analog scale and objectively assessed it using the Drooling Severity Scale and Drooling Frequency Scale. As a result, improvements in the patients' Drooling Severity Scale and Drooling Frequency Scale scores, but no improvements in their visual analog scale scores, were observed after Scopolamine butylbromide treatment. These results indicate that at least some schizophrenic patients with clozapine-induced hypersalivation would benefit from Scopolamine butylbromide treatment.
CONCLUSIONS:
We conclude that clozapine-induced hypersalivation is one factor of stress to patients. Subjective hypersalivation was not improved, but objective hypersalivation was, by Scopolamine butylbromide treatment. However, Scopolamine butylbromide and clozapine possess anticholinergic effects so clinicians should closely monitor patients who take Scopolamine butylbromide.
World J Surg Oncol. 2015 Feb 15;13:50.
Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer.[Pubmed:
25889313]
The aim of this randomized controlled study was to determine whether octreotide (OCT) or Scopolamine butylbromide (SB) was the more effective antisecretive drug controlling gastrointestinal (GI) symptoms due to malignant bowel obstruction (MBO) caused by advanced ovarian cancer.
METHODS AND RESULTS:
Ninety-seven advanced ovarian cancer patients with inoperable MBO were randomized to OCT 0.3 mg/day (OCT group, n = 48) or Scopolamine butylbromide 60 mg/day (Scopolamine butylbromide group, n = 49) for 3 days through a continuous subcutaneous infusion. One patient in the Scopolamine butylbromide group is not included in any assessments since she withdrew consent prior to receiving any treatment because of rapidly progressing cancer. OCT significantly reduced the amount of GI secretions at T1, T2, and T3 (P < 0.05) compared with Scopolamine butylbromide. NGT secretions significantly reduced at T1, T2, and T3 compared with T0 (P < 0.05) in the OCT group, while in the Scopolamine butylbromide group, only at T3, NGT secretions significantly reduced compared with T0. OCT treatment induced a significantly rapid reduction in the number of daily episodes of vomiting and intensity of nausea compared with Scopolamine butylbromide treatment. No significant changes were observed in dry mouth, drowsiness, and colicky pain after either drug. Continuous pain values were significantly lower in the OCT group than in the Scopolamine butylbromide group at T2 and T3 (P < 0.05).
CONCLUSIONS:
At the doses used in this study, OCT was more effective than Scopolamine butylbromide in controlling gastrointestinal symptoms of bowel obstruction. Further studies are necessary to understand the role of hydration more clearly in such a clinical situation.
Masui. 1992 Apr;41(4):670-2.
Scopolamine butylbromide (0.2 mg.kg-1) prevents succinylcholine-induced bradycardia in infants and children.[Pubmed:
1578626]
METHODS AND RESULTS:
We evaluated the effectiveness of Scopolamine butylbromide in preventing succinylcholine-induced bradycardia in infants and children. Forty-two infants and children were randomly assigned into two groups. In group I, 0.2 mg.kg-1 and in group II, 0.4 mg.kg-1 of Scopolamine butylbromide in mixture with succinylcholine (2 mg.kg-1) was administered after halothane induction. HR decreased significantly after halothane induction. Following the injection of the mixture, HR increased above the preinduction value within 20 seconds without any decrease in HR. HR changes were identical in the two groups.
CONCLUSIONS:
In conclusion, Scopolamine butylbromide (0.2 mg.kg-1) was effective in preventing succinylcholine-induced bradycardia in infants and children.
Carcinogenesis. 2011 Sep;32(9):1396-402.
Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice.[Pubmed:
21705482]
M3 subtype muscarinic receptors (CHRM3) are over-expressed in colon cancer.
METHODS AND RESULTS:
In this study, we used Apc(min/+) mice to identify the role of Chrm3 expression in a genetic model of intestinal neoplasia, explored the role of Chrm3 in intestinal mucosal development and determined the translational potential of inhibiting muscarinic receptor activation. We generated Chrm3-deficient Apc(min/+) mice and compared intestinal morphology and tumor number in 12-week-old Apc(min/+)Chrm3(-/-) and Apc(min/+)Chrm3(+/+) control mice. Compared with Apc(min/+)Chrm3(+/+) mice, Apc(min/+)Chrm3(-/-) mice showed a 70 and 81% reduction in tumor number and volume, respectively (P < 0.01). In adenomas, β-catenin nuclear staining was reduced in Apc(min/+)Chrm3(-/-) compared with Apc(min/+)Chrm3(+/+) mice (P < 0.02). Whereas Apc gene mutation increased the number of crypt and Paneth cells and decreased villus goblet cells, these changes were absent in Apc(min/+)Chrm3(-/-) mice. To determine whether pharmacological inhibition of muscarinic receptor activation attenuates intestinal neoplasia, we treated 6-week-old Apc(min/+) mice with Scopolamine butylbromide, a non-subtype-selective muscarinic receptor antagonist. After 8 weeks of continuous treatment, Scopolamine butylbromide-treated mice showed a 22% reduction in tumor number (P = 0.027) and a 36% reduction in tumor volume (P = 0.004) as compared with control mice. Compared with Chrm3 gene ablation, the muscarinic antagonist was less efficacious, most probably due to shorter duration of treatment and incomplete blockade of muscarinic receptors.
CONCLUSIONS:
Overall, these findings indicate that interplay of Chrm3 and β-catenin signaling is important for intestinal mucosal differentiation and neoplasia and provide a proof-of-concept that pharmacological inhibition of muscarinic receptor activation can attenuate intestinal neoplasia in vivo.
J Gastroenterol. 2003;38(7):629-35.
Comparison of gastric peristalsis inhibition by scopolamine butylbromide and glucagon: evaluation by electrogastrography and analysis of heart rate variability.[Pubmed:
12898354]
Activation of glucagon receptors of the smooth muscle membrane suppresses gastric peristalsis. We evaluated autonomic nervous activity by two methods, electrogastrography (EGG) and analysis of heart rate variability, to compare the inhibiting effects of glucagon and Scopolamine butylbromide on gastric peristalsis.
METHODS AND RESULTS:
Heart rate variability, EGG, and blood catecholamine levels were measured before and after administration of glucagon (G group), Scopolamine butylbromide (SB group), or physiological saline (C group). Autonomic nervous function was evaluated using spectral analysis of heart rate variability, and low frequency (LF) and high frequency (HF) power; the LF/HF ratios were also determined.
After administration of Scopolamine butylbromide, HF power, an index of parasympathetic nervous activity, decreased; and the LF/HF ratio, an index of sympathetic nervous activity, increased. In contrast, no significant change was observed in autonomic nervous activity after administration of glucagon. The peak power amplitudes of the EGG decreased significantly in the G and SB groups after intramuscular injection, but the difference between the groups was not significant. Furthermore, the dominant frequency increased significantly in the G and SB groups after injection. Serum catecholamine levels showed no significant changes after administration of Scopolamine butylbromide or glucagon.
CONCLUSIONS:
Inhibition of gastric peristalsis by glucagon via glucagon receptors on smooth muscles did not influence autonomic nervous activity, unlike the results obtained after administration of Scopolamine butylbromide. Therefore, glucagon may be safe for use with elderly patients and those with cardiopulmonary complications.