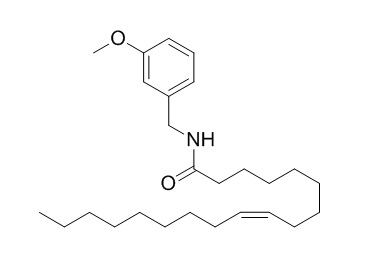
N-(3-Methoxybenzyl)oleamide
Macamides (N-(3-methoxybenzyl)oleamide (MAC 18:1), N-(3-methoxybenzyl)linoleamide (MAC 18:2) and N-(3-methoxybenzyl)linolenamide (MAC 18:3) )achieve their neuroprotective effects by binding to CB1 receptors to protect against Mn-induced toxicity in U-87 MG glioblastoma cells. Additionally these macamides, in a manner similar to the analogous endocannabinoid AEA, interact with other targets such as PPARγ to regulate metabolism and energy homeostasis, cell differentiation and inflammation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules2022, 27(9):2992.
Natural Product Res.&Deve.2022, 1001-6880.
Crystals2020, 10(3), 206.
Korean J. Medicinal Crop Sci.2018, 26(2):148-156
Int. J. Mol. Sci.2022, 23(14),7699;
Molecules.2016, 21(6)
Nutrients.2023, 15(24):5020.
Int J Mol Sci.2024, 25(17):9730.
Int Immunopharmacol.2021, 100:108073.
Nat Prod Sci.2018, 24(3):206
Related and Featured Products
Toxicol Appl Pharmacol, 2018, 340:67-76.
Neuroprotective activity of macamides on manganese-induced mitochondrial disruption in U-87 MG glioblastoma cells.[Reference:
WebLink]
Macamides are a distinct class of secondary metabolites, benzylamides of long chain fatty acids, which were isolated from the Peruvian plant Lepidium meyenii (Maca). As structural analogues of the endocannabinoid anandamide (AEA), they have demonstrated neuroprotective effects in vitro and in vivo. The purpose of this study was to demonstrate the neuroprotective activity of the macamides: N-(3-Methoxybenzyl)oleamide (MAC 18:1), N-(3-methoxybenzyl)linoleamide (MAC 18:2) and N-(3-methoxybenzyl)linolenamide (MAC 18:3) in a neurotoxic environment caused by exposure of U-87 MG glioblastoma cells to manganese chloride (MnCl2). The neuroprotective effects of these macamides were reversed by the CB1 antagonist AM251.
METHODS AND RESULTS:
The mechanism by which manganese (Mn) induces cell damage was investigated by studying its effects on mitochondria. Reactive oxygen species (ROS) increase intracellular calcium and enhance the opening of mitochondrial permeability transition pores (MPTP), which leads to decreased mitochondrial membrane potential (MMP), to disruption of mitochondria and to neuron death in neurodegenerative disorders. In this study, MnCl2 at 50 μM was responsible for mitochondrial disruption, which was attenuated by all three of the macamides tested. Human peroxisome proliferator-activated receptor gamma (PPARγ) has been proposed to be a cannabinoid target, and PPARγ has also been demonstrated to mediate some of the longer-term vascular effects of the plant cannabinoid, ∆ 9-tetrahydrocannabinol. PPARγ activation was observed in response to exposures of cells to MAC 18:2 and MAC 18:3.
CONCLUSIONS:
These findings suggest that macamides achieve their neuroprotective effects by binding to CB1 receptors to protect against Mn-induced toxicity in U-87 MG glioblastoma cells. Additionally these macamides, in a manner similar to the analogous endocannabinoid AEA, interact with other targets such as PPARγ to regulate metabolism and energy homeostasis, cell differentiation and inflammation.