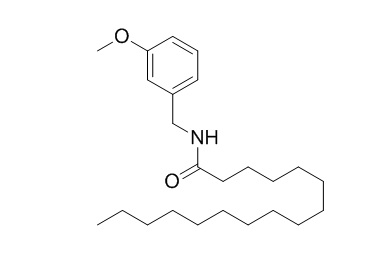
N-(3-Methoxybenzyl)palmitamide
N-(3-methoxybenzyl)palmitamide is a promising inhibitor of fatty acid amide hydrolase (FAAH) and could potentially offer a good alternative for the treatment of pain, inflammation and CNS degenerative disorders. N-(3-Methoxybenzyl)palmitamide can counteract the toxicity produced by Aβ (25-35).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Advances in Traditional Medicine 2021, 21:779-789.
Int J Mol Sci.2023, 24(3):2102.
Nutrients.2019, 12(1):E40
Huazhong Agricultural University2022, pp34.
BMC Cancer. 2021, 21(1):91.
Comput Biol Med.2024, 178:108775.
Toxins (Basel).2022, 14(12):824.
Food Sci Nutr.2023, 11(9):5532-5542.
Nutrients.2023, 15(13):2960.
Toxicol In Vitro.2024, 99:105876.
Related and Featured Products
Life Sciences, 1992 , 51 (10) :779-86.
Neuroprotective effects of Lepidium meyenii (Maca) and macamides against amyloid-beta (25-35) induced toxicity in B-35 neuroblastoma cells (657.13)[Reference:
WebLink]
The purpose of this study was to evaluate the neuroprotective effects of a Maca pentane extract (5-50 μg/mL) and some of the component macamides: N-benzylpalmitamide, N-benzyloleamide and N-(3-Methoxybenzyl)palmitamide (1-30 μM) on the B-35 neuroblastoma cell line.
METHODS AND RESULTS:
B-35 cells were pre-treated with extract or macamides and subsequently exposed to a neurotoxic concentration (10 μM) of Aβ (25-35). A cell viability assay utilizing 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was performed to evaluate the neuroprotective effects. The results demonstrated that the Maca extract and N-(3-Methoxybenzyl)palmitamide counteracted the toxicity produced by Aβ (25-35), demonstrating significant increases in cell viability (34% and 21% respectively). Furthermore, when the tested compounds were evaluated for antioxidant activity and their effects on caspase 3, neither the Maca extract nor N-(3-Methoxybenzyl)palmitamide demonstrated an antioxidant effect or caspase 3 inhibition.
CONCLUSIONS:
These results suggest that the Maca extract and macamides may produce neuroprotective effects against Aβ by mechanisms other than caspase 3 inhibition or an antioxidant effect.
Le Chirurgien-dentiste de France , 1984 , 53 (228) :33-6.
Inhibitory effect of macamides: N-benzylpalmitamide and N-(3-methoxybenzyl)palmitamide on fatty acid amide hydrolase (FAAH)[Reference:
WebLink]
METHODS AND RESULTS:
Each compound was tested at concentrations from 1 to 100 μM, using an FAAH inhibitory activity assay, which is a fluorescence-based method.
The results demonstrated that each of the test compounds causes a concentration-dependent inhibition of FAAH. The pre-incubation study revealed that N-benzylpalmitamide and N-(3-Methoxybenzyl)palmitamide inhibit FAAH in a time-dependent manner. The % inhibition of FAAH produced by 100 μM N-(3-Methoxybenzyl)palmitamide without pre-incubation was comparable to that of N-benzylpalmitamide. However, a 120 min pre-incubation of inhibitor with FAAH caused a significant increase in the % inhibition produced by 100 μM N-(3-Methoxybenzyl)palmitamide. The enzyme kinetics study indicated that N-(3-Methoxybenzyl)palmitamide is likely an uncompetitive inhibitor of FAAH. LC-MS/MS analysis determined that N-(3-Methoxybenzyl)palmitamide is a substrate of FAAH since it undergoes hydrolysis by FAAH.
METHODS AND RESULTS:
The results of this study indicated that N-(3-Methoxybenzyl)palmitamide is a promising inhibitor of FAAH and could potentially offer a good alternative for the treatment of pain, inflammation and CNS degenerative disorders.
Glyasperin C
Catalog No: CFN95065
CAS No: 142474-53-1
Price: $333/10mg
5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No: CFN95084
CAS No: 141846-47-1
Price: $413/5mg
5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No: CFN95137
CAS No: 105955-04-2
Price: $318/10mg
Dehydrojuncusol
Catalog No: CFN95143
CAS No: 117824-04-1
Price: $318/5mg
Puerol B
Catalog No: CFN95155
CAS No: 112343-17-6
Price: $398/5mg
4,4-di(4-hydroxy-3-methoxyphenly)-2,3-dimethylbutanol
Catalog No: CFN95200
CAS No: 913643-31-9
Price: $368/5mg
3-Oxo-4-benzyl-3,4-dihydro-1H-pyrrolo [2,1-c] oxazine-6-methylal
Catalog No: CFN95293
CAS No: 60026-28-0
Price: $413/5mg
4'-O-Methyllucenin II (Diosmetin 6,8-di-C-glucoside)
Catalog No: CFN95344
CAS No: 98813-28-6
Price: $318/10mg
New compound 13
Catalog No: CFN95374
CAS No: N/A
Price: $318/5mg
3-Oxo-alpha-ilexanolic acid
Catalog No: CFN95496
CAS No: N/A
Price: $413/5mg