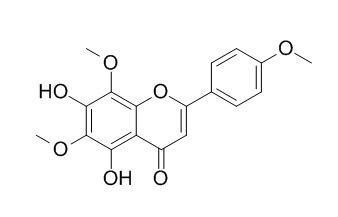
Lysionotin
Lysionotin is a natural flavonoid predominantly found in fewflower lysionotus herbs and possesses many pharmacological properties, such as antibacterial, anti-inflammatory, antihypertensive, and free radical scavenging activities. It is an efficient inhibitor of α-toxin expression and shows significant protection against S. aureus in vitro and in vivo.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutr Res Pract2019, 13:e45
Foods.2023, 12(6):1227.
Life Sci.2019, 216:259-270
Acta Physiologiae Plantarum2016, 38:7
Antioxidants (Basel).2020, 9(4):284.
Srinakharinwirot University2023, 2669.
Nutrients.2019, 12(1):E40
Geroscience.2024, 01207-y.
J Appl Toxicol.2020, 40(7):965-978.
Appl. Sci.2020, 10(4),1304
Related and Featured Products
Appl Microbiol Biotechnol. 2017 Sep;101(17):6697-6703.
Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting α-toxin expression.[Pubmed:
28710557 ]
α-Toxin, one of the best known pore-forming proteins produced by Staphylococcus aureus (S. aureus), is a critical virulence factor in multiple infections. The necessity of α-toxin for S. aureus pathogenicity suggests that this toxin is an important target for the development of a potential treatment strategy.
METHODS AND RESULTS:
In this study, we showed that Lysionotin, a natural compound, can inhibit the hemolytic activity of culture supernatants by S. aureus by reducing α-toxin expression. Using real-time PCR analysis, we showed that transcription of hla (the gene encoding α-toxin) and agr (the locus regulating hla) was significantly inhibited by Lysionotin. Lactate dehydrogenase and live/dead assays indicated that Lysionotin effectively protected human alveolar epithelial cells against S. aureus, and in vivo studies also demonstrated that Lysionotin can protect mice from pneumonia caused by S. aureus. These findings suggest that Lysionotin is an efficient inhibitor of α-toxin expression and shows significant protection against S. aureus in vitro and in vivo.
CONCLUSIONS:
This study supports a potential strategy for the treatment of S. aureus infection by inhibiting the expression of virulence factors and indicates that Lysionotin may be a potential treatment for S. aureus pneumonia.
Appl Microbiol Biotechnol. 2017 Sep;101(17):6697-6703.
Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting α-toxin expression.[Pubmed:
28710557 ]
Cell lines:A549 human lung epithelial cells
Concentrations: 0-16 μg/ml
Incubation Time: 5 h
Method:
A549 human lung epithelial cells (ATCC CCL 185) are maintained in DMEM supplemented with 10% fetal serum at 37 ℃ and seeded in 96-well plates at a density of approximately 2 × 104 cells per well. Then, the cells are cultured with 200 μl of bacteria with various concentrations of Lysionotin and incubated for 5 h at 37 ℃. For LDH assays, the supernatants from wells are assessed for LDH release with the Cytotoxicity Detection Kit.
Appl Microbiol Biotechnol. 2017 Sep;101(17):6697-6703.
Lysionotin attenuates Staphylococcus aureus pathogenicity by inhibiting α-toxin expression.[Pubmed:
28710557 ]
Animal Models: 6- to 8-week-old female C57BL/6J mice
Formulation: DMSO
Dosages:100 mg/kg
Administration: by hypodermic injection in the back skin
Acta Pharmacol Sin. 2002 Jul;23(7):667-72.
Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects.[Pubmed:
12100765]
To study the relationship between the structure and hydroxyl radical (*OH)-scavenging activity of twelve natural flavonoids.
METHODS AND RESULTS:
The hydroxyl radical-generating chemiluminescence system with ascorbate-CuSO4-yeast-H2O2 was used to determine the hydroxyl radical-scavenging activity of twelve natural flavonoids.
Guercetin, heliosin, hyperoside, kaempferol, baicalin, corylifolin, Lysionotin, matteucinol, corylifolinin, and genistein could effectively scavenge. OH and inhibit the chemiluminescence of the system. The IC50 values (95 % confidence limits) of the flavonoids were 12.1 (9.9-14.5) g/L, 15.8(14.0-19.2) g/L, 19.5 (16.8-27.4) g/L, 20.1 (13.6-29.0) g/L, 34.6 (28.4-43.4) g/L, 66.8 (63.2-74.4) g/L, 187 (147-235) g/L, 211 (165-284) g/L, 262 (190-346) g/L, and 708 (498-994) g/L, respectively; whereas nobilelin and corylifolin-Ac could not scavenge *OH.
CONCLUSIONS:
(1) Phenolic hydroxyls in flavonoids were the main active groups capable of scavenging *OH; (2) Hydroxyl groups in ring B and A were important *OH-scavenging active groups; (3) The ortho-dihydroxyl groups in ring A and/or B could greatly enhance the *OH-scavenging activity of the rings; (4) Comparing the IC50 values of guercetin, heliosin, hyperoside, baicalin, Lysionotin, and matteucinol, it was suggested that the hydroxyl groups on 3',4' position of ring B possessed high *OH-scavenging activity and the scavenging activity of hydroxyl groups in ring B was higher than that of hydroxyl groups in ring A. The hydroxyl group or glucoside on 3 position of ring C of the above mentioned 6 flavonoids was also related to the. OH-scavenging ability. (5) The structural types of flavonoids themselves could influence their *OH-scavenging activity.