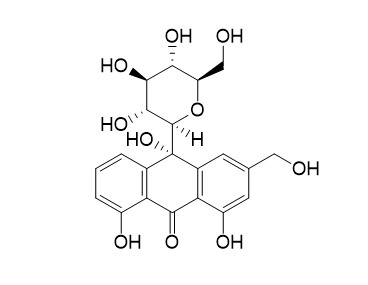
10-Hydroxyaloin B
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep.2020, 10:4495(2020)
Environ Toxicol.2023, 38(7):1641-1650.
Nat Commun.2025, 16(1):4121.
Malaysian Journal of Analytical Sciences2022, 26(2):360-369.
Acta Edulis Fungi2020, 27(02):63-76.
Front Pharmacol.2021, 12:770667.
Industrial Crops and Products2022, 188:115596.
Heliyon.2024, 10(16):e35645.
J Clin Med.2019, 8(10):E1664
J AOAC Int.2023, 106(1):56-64.
Related and Featured Products
Planta Med . 1992 Jun;58(3):259-262.
Structure revision of 4-hydroxyaloin: 10-hydroxyaloins a and B as main in vitro-oxidation products of the diastereomeric aloins1[Pubmed:
17226466]
A reinvestigation of the anthrone derivative 4-hydroxyaloin as main product of the mild IN VITRO-oxidation of aloin has led to the revision of the proposed structure as the aloin-analogous oxanthrone derivative, the new 10-hydroxyaloin, which was prepared from aloin by an improved procedure in ammonia at pH 9. 10-Hydroxyaloin was separated into its C10-diastereomers A and B by analytical and preparative chromatographic methods. Their structures were elucidated by spectroscopic methods (FAB-MS; (1)H/ (13)C-NMR; CD), which show that 10-hydroxyaloin A is the 10 R,1' R compound and that 10-Hydroxyaloin B has the 10 S,1' R-configuration.
Phytochem Anal . Sep-Oct 2003;14(5):275-280.
Identification of major metabolites in Aloe littoralis by high-performance liquid chromatography-nuclear magnetic resonance spectroscopy[Pubmed:
14515998]
Examination of the leaf exudate of the South African species Aloe littoralis by reversed-phase HPLC revealed the presence of two major metabolites. The identification of the two compounds without isolation was attempted by HPLC-NMR based on separation using a C18 column eluting with a deuterium oxide:acetonitrile solvent gradient and an inverse HPLC-NMR probe. For each compound, one-dimensional proton spectra, and two-dimensional homonuclear COSY and TOCSY, and heteronuclear HSQC and HMBC, spectra were collected. On the basis of the data obtained, the metabolites were characterised as 10-Hydroxyaloin B and deacetyllittoraloin.