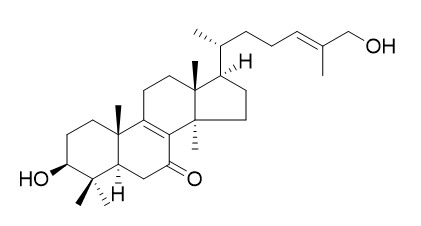
Lucidadiol
Lucidadiol exhibits butyrylcholinesterase-inhibitory activity at concentrations up to 200 uM, it also possesses inhibitory activity against acetylcholinesterase (AChE) with the percentage inhibition at 100 uM. Lucidadiol shows antiviral activity against influenza virus type A and HSV type 1.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules2022, 27(11):3606.
BMC Complement Altern Med.2019, 19(1):367
Nat Commun.2021, 12(1):681.
Pharmaceuticals (Basel).2021, 14(6):588.
Korean Journal of Pharmacognosy2017, 48(4):320-328
Am J Chin Med.2016, 44(8):1719-1735
Pharmaceutics.2021, 13(7):1028.
Ind. J. Pharm. Edu. Res.2023; 57(3):1132-1139.
Chemistry of Plant Raw Materials2019, 4:135-147
Research Square2021, March 3rd.
Related and Featured Products
Phytochemistry. 2015 Feb;110:133-9.
Lanostanoids with acetylcholinesterase inhibitory activity from the mushroom Haddowia longipes.[Pubmed:
25577284 ]
METHODS AND RESULTS:
Nine lanostanoids, together with nine known ones, were isolated from the ethyl acetate extract of the fruiting bodies of the mushroom Haddowia longipes. Their structures were elucidated as 11-oxo-ganoderiol D, lanosta-8-en-7,11-dioxo-3β-acetyloxy-24,25,26-trihydroxy, lanosta-8-en-7-oxo-3β-acetyloxy-11β,24,25,26-tetrahydroxy, lanosta-7,9(11)-dien-3β-acetyloxy-24,25,26-trihydroxy, lanosta-7,9(11)-dien-3β-acetyloxy-24,26-dihydroxy-25-methoxy, 11-oxo-Lucidadiol, 11β-hydroxy-Lucidadiol, lucidone H and lanosta-7,9(11),24E-trien-3β-acetyloxy-26,27-dihydroxy by analysing their 1D/2D NMR and MS spectra.
CONCLUSIONS:
In addition, bioassays of inhibitory activity against acetylcholinesterase (AChE) of all compounds showed that thirteen compounds possessed inhibitory activity against AChE with the percentage inhibition ranging from 10.3% to 42.1% when tested at 100 μM.
Bioorg Med Chem Lett. 2011 Nov 1;21(21):6603-7.
Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum.[Pubmed:
21924611 ]
METHODS AND RESULTS:
Two new lanostane triterpenes, named methyl ganoderate A acetonide (1) and n-butyl ganoderate H (2), were isolated from the fruiting bodies of Ganoderma lucidum together with 16 known compounds (3-18). Extensive spectroscopic and chemical studies established the structures of these compounds as methyl 7β,15α-isopropylidenedioxy-3,11,23-trioxo-5α-lanost-8-en-26-oate (1) and n-butyl 12β-acetoxy-3β-hydroxy-7,11,15,23-tetraoxo-5α-lanost-8-en-26-oate (2). Because new compounds exhibiting specific anti-acetylcholinesterase activity are being sought as possible drug candidates for the treatment of Alzheimer's and related neurodegenerative diseases, compounds 1-18 were examined for their inhibitory activities against acetylcholinesterase and butyrylcholinesterase. All of the compounds exhibited moderate acetylcholinesterase-inhibitory activity, with IC(50) values ranging from 9.40 to 31.03μM. In contrast, none of the compounds except Lucidadiol (13) and lucidenic acid N (14) exhibited butyrylcholinesterase-inhibitory activity at concentrations up to 200μM.
CONCLUSIONS:
These results indicate that these lanostane triterpenes are preferential inhibitors of acetylcholinesterase and may be suitable drug candidates.
Fitoterapia. 2003 Feb;74(1-2):177-80.
Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi.[Pubmed:
12628419]
Ganodermadiol, Lucidadiol and applanoxidic acid G were isolated as first triterpenes from the European Basidiomycete Ganoderma pfeifferi. The compounds show antiviral activity against influenza virus type A and HSV type 1.
2''-O-Galloylquercitrin
Catalog No: CFN95041
CAS No: 80229-08-9
Price: $418/5mg
Isololiolide
Catalog No: CFN95106
CAS No: 38274-00-9
Price: $318/5mg
3,7,23,24-tetrahydroxycucurbita-5,25-dien-19-al
Catalog No: CFN95168
CAS No: 1446447-97-7
Price: $318/5mg
5-Hydroxy-7,8-dimethoxy (2R)-flavanone-5-O-beta-D-glucopyranoside
Catalog No: CFN95209
CAS No: 942626-74-6
Price: $318/10mg
Dipsacussaponin C
Catalog No: CFN95353
CAS No: 152406-43-4
Price: $318/5mg
(Z)-3,11-dimethy-7-methylene-9,14-epoxy-1,6,10-dodecatrien-3-ol
Catalog No: CFN95402
CAS No: 1392202-57-1
Price: $318/10mg
12beta-Acetoxy-3beta-hydroxy-7,11,15,23-tetraoxo-lanost-8,20-diene-26-oic acid
Catalog No: CFN95468
CAS No: 1085338-75-5
Price: $318/5mg
Ganoderic acid GS-2
Catalog No: CFN95549
CAS No: 1206781-65-8
Price: $413/5mg
Mahuannin B
Catalog No: CFN95554
CAS No: 82796-37-0
Price: $318/5mg
Resinacein D
Catalog No: CFN95591
CAS No: 2231061-47-3
Price: $413/5mg