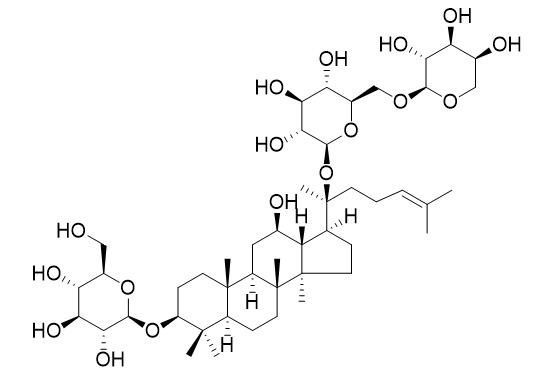
Ginsenoside Rd2 (Quinquenoside L10)
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2022, 23(5):2796.
J Appl Biol Chem2022, 65:343−348.
Korean Journal of Pharmacognosy2014, 113-120
Food Chem.2018, 252:207-214
Sci Rep.2019, 9(1):6429
Natural Product Communications2022, 7(3):1-7.
J Ethnopharmacol.2025, 350:120002.
Plant Archives2020, 2(1),2929-2934
Separations2023, 10(4), 231.
Molecules.2022, 27(7):2093.
Related and Featured Products
Modern chinese medicine, 2018.
Saponin Constituents from Fruits of Panax ginseng.[Reference:
WebLink]
To study the saponin constituents of in the fruits of Panax ginseng C. A. Meyer.
METHODS AND RESULTS:
The chemical constituents were isolated and purified by various chromatographic methods including macroporous resin,MCI gel, silica gel columns and semi-preparative HPLC, and their structures were identified by NMR and MS data analysis. Twenty-two compounds were isolated and identified as ginsenoside F2(1),vina-ginsenoside R8(2),pseudoginsenoside Rc1(3),ginsenoside Re(4),notoginsenoside R2(5),ginsenoside F5(6),ginsenoside F3(7),ginsenoside Ia(8),chikusetsusaponin LM1(9),20(S)-ginsenoside Rg2(10),ginsenoside Rc(11),ginsenoside F1(12),ginsenoside Rd(13),ginsenoside Rb2(14),ginsenoside Rb1(15),ginsenoside Rh6(16),ginsenoside Rh4(17),ginsenoside Rh5(18),gypenoside XⅦ(19),gypenoside IX(20),notoginsenoside Fe(21) and Ginsenoside Rd2 (Quinquenoside L10)(22).
CONCLUSIONS:
Compounds 2,3,5,8,9,16,18-22 were isolated from the fruits of P. ginseng for the first time.
Journal of Asian Natural Products Research, 2009, 11(3):195-201.
Three new triterpenoid saponins from the leaves and stems of Panax quinquefolium.[Reference:
WebLink]
Three new minor dammarane saponins, Ginsenoside Rd2 (Quinquenoside L10) (1), L14 (2), and L16 (3), were isolated from the leaves and stems of Panax quinquefolium L.
METHODS AND RESULTS:
By the combination of one- and two-dimensional NMR techniques and MS spectroscopic analysis, their structures were established as 20-O-(alpha-L-arabinopyranosyl-(1-6)-O-beta-D-glucopyranosyl)-3-O-beta-D-glucopyranosyl-dammar-24-ene-3,12, 20-triol (1), 20-O-alpha-L-arabinopyranosyl-3-O-(beta-D-glucopyranosyl-(1-2)-O-beta-d-glucopyranosyl)-dammar-24-ene-3,12,20-triol (2), 3-O-(beta-D-glucopyranosyl-(1-2)-O-beta-d-glucopyranosyl)-20-O-(beta-D-glucopyranosyl-(1-6)-beta-D-glucopyranosyl)-dammarane-3,12,20,24,25-pentaol (3).
CONCLUSIONS:
The content of artemisinin was significantly enhanced by 3.0 mg l(- 1) of compounds 1, 2, and 3 in the callus of Artemisia annua.