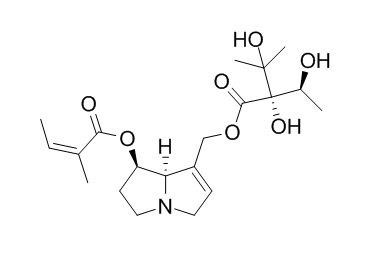
Echimidine
Echimidine, a major hepatotoxic dehydropyrrolizidine alkaloid produced by E. plantagineum, in the honey (780 ng/g) and in the subsequent mead samples (236–540 ng/mL). Echimidine-N-Oxide has antifungal activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean J. Medicinal Crop Sci.2023, 31(6):388-395.
J Pharm Biomed Anal.2019, 172:268-277
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):1-20.
Plos One.2020, 10.1371
Front Pharmacol.2018, 9:236
Front Microbiol.2019, 10:2806
PLoS One.2018, 13(11):e0208055
Int J Mol Sci.2024, 25(17):9730.
Metabolites.2020, 10(12):497.
Chengdu University of Traditional Chinese Medicine2024, 4802935.
Related and Featured Products
Mol Nutr Food Res. 2014 May;58(5):995-1004.
Structure-activity relationship in the passage of different pyrrolizidine alkaloids through the gastrointestinal barrier: ABCB1 excretes heliotrine and echimidine.[Pubmed:
24375927]
1,2-Unsaturated pyrrolizidine alkaloids (PA) are found in plants such as Asteraceae and Boraginaceae families. Acute PA poisoning via contaminated food or feed causes severe damage to liver depending on species-specific oral bioavailability. For assessing PA bioavailability, their passage across the intestinal barrier was investigated using Caco-2 cells.
METHODS AND RESULTS:
Differentiated Caco-2 cells were exposed in transport chambers to the PA heliotrine (Hn), Echimidine (Em), senecionine (Sc), and senkirkine (Sk). Cell supernatants were analyzed by LC-MS/MS.
PA pass Caco-2 monolayer from the apical into basolateral compartment depending on their chemical structure. Compared to the cyclic diesters Sc and Sk with a passage rate of 47% ± 4 and 40% ± 3, respectively, the transferred amount of the monoester Hn (32% ± 3) and open-chained diester Em (13% ± 2) was substantially lower. This suggested an active transport of Hn and Em. Using Madin-Darby canine kidney II/P-glycoprotein (ABCB1)-overexpressing cells, the active excretion of Hn and Em by ABCB1 from the gastrointestinal epithelium into the gut lumen was shown.
CONCLUSIONS:
PA cross the intestinal barrier structure-dependently. The passage of the noncyclic PA Hn and Em is reduced by an ABCB1-driven efflux into the gastrointestinal lumen resulting in a decreased oral bioavailability.
J.Food Compos. Anal., 2013, 29(2):106-9.
Persistence of echimidine, a hepatotoxic pyrrolizidine alkaloid, from honey into mead[Reference:
WebLink]
Honey produced by bees foraging on Echium plantagineum is known to contain dehydropyrrolizidine alkaloids characteristic of the plant.
METHODS AND RESULTS:
Following a prolific growth of E. plantagineum in the wake of Australian bushfires, two samples of mead, a fermented drink made from honey, and the honey used to prepare the mead were analyzed for the presence of Echium-related dehydropyrrolizidine alkaloids. HPLC–esiMS and MS/MS analysis of the alkaloidal fractions obtained using strong cation exchange, solid phase extraction unequivocally confirmed the presence of Echimidine, a major hepatotoxic dehydropyrrolizidine alkaloid produced by E. plantagineum, in the honey (780 ng/g) and in the subsequent mead samples (236–540 ng/mL).
CONCLUSIONS:
The results from this limited, and specifically targeted sample set, while not indicative of the extent of the presence of Echimidine (or other dehydropyrrolizidine alkaloids) in meads, reinforce the need for a wider survey and perhaps subsequent routine monitoring to determine the potential contribution to long-term, low-level or intermittent exposure to these toxic alkaloids and consequent chronic disease development.
Turk. J. Med. Sci., 2001, 31(6):487-92.
Antifungal activities of different extracts and echimidine-N-oxide from Symphytum sylvaticum Boiss. subsp. sepulcrale (Boiss. & Bal.) Greuter & Burdet var. sepulcrale.[Reference:
WebLink]
METHODS AND RESULTS:
Antifungal activity was determined by the tube dilution method on different extracts of Symphytum sylvaticum Boiss. subsp. sepulcrale (Boiss. & Bal.) Greuter & Burdet var. sepulcrale. The antifungal activity of the isolated compound from the root alkaloid fraction (Echimidine-N-Oxide) was also tested against ten fungal cultures.
CONCLUSIONS:
The activity was found to be mainly due to Echimidine-N-Oxide (ENO). The quantitative determination of ENO was also carried out with capillary gas chromatography in the roots and the aerial parts of the plant.
Toxicol In Vitro. 2015 Jun 20;29(7):1669-1682.
Disturbance of gene expression in primary human hepatocytes by hepatotoxic pyrrolizidine alkaloids: A whole genome transcriptome analysis.[Pubmed:
26100227 ]
1,2-unsaturated pyrrolizidine alkaloids (PA) are plant metabolites predominantly occurring in the plant families Asteraceae and Boraginaceae. Acute and chronic PA poisoning causes severe hepatotoxicity. So far, the molecular mechanisms of PA toxicity are not well understood.
METHODS AND RESULTS:
To analyze its mode of action, primary human hepatocytes were exposed to a non-cytotoxic dose of 100 μM of four structurally different PA: Echimidine, heliotrine, senecionine, senkirkine. Changes in mRNA expression were analyzed by a whole genome microarray. Employing cut-off values with a |fold change| of 2 and a q-value of 0.01, data analysis revealed numerous changes in gene expression. In total, 4556, 1806, 3406 and 8623 genes were regulated by Echimidine, heliotrine, senecione and senkirkine, respectively. 1304 genes were identified as commonly regulated. PA affected pathways related to cell cycle regulation, cell death and cancer development. The transcription factors TP53, MYC, NFκB and NUPR1 were predicted to be activated upon PA treatment. Furthermore, gene expression data showed a considerable interference with lipid metabolism and bile acid flow. The associated transcription factors FXR, LXR, SREBF1/2, and PPARα/γ/δ were predicted to be inhibited.
CONCLUSIONS:
In conclusion, though structurally different, all four PA significantly regulated a great number of genes in common. This proposes similar molecular mechanisms, although the extent seems to differ between the analyzed PA as reflected by the potential hepatotoxicity and individual PA structure.