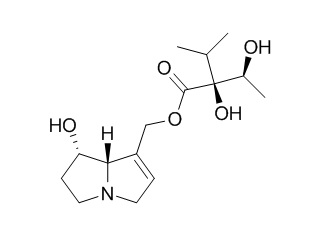
Echinatine
Echinatin exerts a protective effect against ischemia/reperfusion (I/R)-induced myocardial injury on hearts, this effect may be attributed to the antioxidant and anti-inflammatory activities of this compound. Echinatin can inhibit DNP-ATPase activity while stimulating range latent ATPase activity in the low concentration, so echinatin can disturb the mitochondrial energy transfer reactions and membrane permeability.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Med Rep.2015, 12(5):7789-95
Cancers (Basel).2023, 15(1):37.
Antioxidants (Basel).2022, 11(12):2411.
Int J Mol Sci.2021, 22(10):5181.
Food Research International2020, 108987
Nutrients.2019, 11(4):E936
Sichuan Agricultural University2023, 4630743.
Nat Prod Sci.2018, 24(3):206
Int J Med Sci.2024, 21(15):2883-2896
Front Cell Dev Biol.2020, 8:32.
Related and Featured Products
J.Toxicol. Sci., 1982, 7(4):245-54.
The effects of echinatin and its related compounds on the mitochondrial energy transfer reaction.[Pubmed:
6221118]
METHODS AND RESULTS:
To investigate the mechanism by which various biological action of licorice root are brought about, the effects of echinatin(Echinatine) as a small constituent of Glycyrrhiza echinata and several related compounds on mitochondrial energy transfer reactions were examined. The results obtained were as follows: 1) Echinatin, 4'-hydroxychalcone, chalcone and 3,4'-dihydroxychalcone at a low concentration cause deterioration of respiratory control and oxidative phosphorylation of isolated rat liver mitochondria. 2) Chalcone and 4'-hydroxychalcone stimulate both latent and DNP-ATPase activity of mitochondria. Echinatin inhibits DNP-ATPase activity while stimulating range latent ATPase activity in the low concentration. 3) Chalcone and 4'-hydroxychalcone induce a rapid potassium release from mitochondrial vesicles, while echinatin and 3,4'-dihydroxychalcone have lesser effect than the former two substances.
CONCLUSIONS:
From these results, it can be concluded that echinatin and several related compounds disturb the mitochondrial energy transfer reactions and membrane permeability.
BMC Cardiovasc Disord. 2016 May 31;16:119.
Cardioprotection provided by Echinatin against ischemia/reperfusion in isolated rat hearts.[Pubmed:
27246834]
This study evaluated the protective effect of Echinatin(Echinatine) against myocardial ischemia/reperfusion (I/R) injury in rats.
METHODS AND RESULTS:
The effect of Echinatin(Echinatine) on cardiac function in rats subjected to I/R was demonstrated through improved Langendorff retrograde perfusion technology. Adult Sprague-Dawley rats were randomly divided into five groups, and myocardial infarct size was macroscopically estimated through 2,3,5-triphenyltetrazolium chloride staining. The coronary effluent was analyzed for the release of lactate dehydrogenase (LDH) and creatine kinase (CK) to assess the degree of cardiac injury. The concentrations of malondialdehyde (MDA), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were determined along with superoxide dismutase (SOD) activity using ELISA. Finally, cardiomyocyte apoptosis analysis was conducted with POD, an in situ cell death detection kit. Echinatin (Echinatine,0.5 and 2.5 μg/mL) pretreatment enhanced the maximum up/down rate of the left ventricular pressure (±dp/dtmax), improved the heart rate, increased the left ventricular developed pressure (LVDP), enhanced the coronary flow, and reduced the CK and LDH levels in the coronary flow of the treated group compared with the I/R group. Echinatin limited the contents of CK and LDH, improved the LVDP, reduced the contents of MDA, IL-6, and TNF-α, and increased the SOD activity. The infarct size and cell apoptosis in the hearts of the rats in the Echinatin(Echinatine)-treated group were smaller and lower, respectively, than those in the hearts of the rats in the I/R control group.
CONCLUSIONS:
Echinatin(Echinatine) exerts a protective effect against I/R-induced myocardial injury on hearts. This effect may be attributed to the antioxidant and anti-inflammatory activities of this compound.
Phytochem Anal. 2014 Sep-Oct;25(5):429-38.
Semi-automated separation of the epimeric dehydropyrrolizidine alkaloids lycopsamine and intermedine: preparation of their N-oxides and NMR comparison with diastereoisomeric rinderine and echinatine.[Pubmed:
24816769]
The diversity of structure and, particularly, stereochemical variation of the dehydropyrrolizidine alkaloids can present challenges for analysis and the isolation of pure compounds for the preparation of analytical standards and for toxicology studies.
To investigate methods for the separation of gram-scale quantities of the epimeric dehydropyrrolizidine alkaloids lycopsamine and intermedine and to compare their NMR spectroscopic data with those of their heliotridine-based analogues Echinatine and rinderine.
METHODS AND RESULTS:
Lycopsamine and intermedine were extracted, predominantly as their N-oxides and along with their acetylated derivatives, from commercial samples of comfrey (Symphytum officinale) root. Alkaloid enrichment involved liquid-liquid partitioning of the crude methanol extract between dilute aqueous acid and n-butanol, reduction of N-oxides and subsequent continuous liquid-liquid extraction of free base alkaloids into CHCl3 . The alkaloid-rich fraction was further subjected to semi-automated flash chromatography using boronated soda glass beads or boronated quartz sand.
Boronated soda glass beads (or quartz sand) chromatography adapted to a Biotage Isolera Flash Chromatography System enabled large-scale separation (at least up to 1-2 g quantities) of lycopsamine and intermedine. The structures were confirmed using one- and two-dimensional (1) H- and (13) C-NMR spectroscopy. Examination of the NMR data for lycopsamine, intermedine and their heliotridine-based analogues Echinatine and rinderine allowed for some amendments of literature data and provided useful comparisons for determining relative configurations in monoester dehydropyrrolizidine alkaloids. A similar NMR comparison of lycopsamine and intermedine with their N-oxides showed the effects of N-oxidation on some key chemical shifts. A levorotatory shift in specific rotation from +3.29° to -1.5° was observed for lycopsamine when dissolved in ethanol or methanol respectively.
CONCLUSIONS:
A semi-automated flash chromatographic process using boronated soda glass beads was standardised and confirmed as a useful, larger scale preparative approach for separating the epimers lycopsamine and intermedine. The useful NMR correlations to stereochemical arrangements within this specific class of dehydropyrrolizidine alkaloid cannot be confidently extrapolated to other similar dehydropyrrolizidine alkaloids.