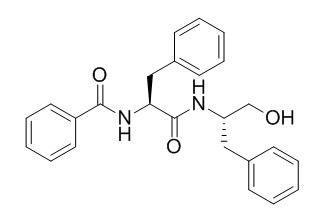
Aurantiamide
Aurantiamide acetate has anti-cancer, anti-inflammatory and antinociceptive activities, it may suppress the growth of malignant gliomas by blocking autophagic flux, it also inhibits cysteine proteinases, in particular, cathepsin L (3.4.22.15) and B (3.4.22.1) with IC50 of 12 microM and 49 microM, respectively . Aurantiamide acetate has an anti-neuroinflammatory effect on LPS stimulation through its inhibition of the NF-κB, JNK and p38 pathways.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J.Pharm. & Biome. Anal.2023, 2: 100018.
Chem Biol Interact.2020, 328:109200.
Molecules.2019, 24(22):E4022
Drug Chem Toxicol.2020, 1-14.
Molecules.2024, 29(23):5792.
Natural Product Sciences2023, 29(4):276-280.
Processes2020, 8(12),1540.
Food Funct.2022, 13(13):6923-6933.
Sci Rep.2019, 9:19059
Phytomedicine2022, 104:154337.
Related and Featured Products
Int Immunopharmacol. 2014 Dec;23(2):568-74.
Anti-neuroinflammatory effect of aurantiamide acetate from the marine fungus Aspergillus sp. SF-5921: inhibition of NF-κB and MAPK pathways in lipopolysaccharide-induced mouse BV2 microglial cells.[Pubmed:
25448500]
METHODS AND RESULTS:
In the course of a search for anti-neuroinflammatory metabolites from marine fungi, Aurantiamide acetate (1) was isolated from marine-derived Aspergillus sp. as an anti-neuroinflammatory component. Compound 1 dose-dependently inhibited the production of nitric oxide (NO) and prostaglandin E2 (PGE2) in BV2 microglial cells. It also attenuated inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2), and other pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). In a further study designed to elucidate the mechanism of its anti-neuroinflammatory effect, Aurantiamide acetate was shown to block the activation of nuclear factor-kappa B (NF-κB) in lipopolysaccharide (LPS)-induced BV2 microglial cells by inhibiting the phosphorylation of the inhibitor kappa B-α (IκB)-α. In addition, Aurantiamide acetate decreased the phosphorylation levels of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (MAPKs).
CONCLUSIONS:
These results suggest that Aurantiamide acetate has an anti-neuroinflammatory effect on LPS stimulation through its inhibition of the NF-κB, JNK and p38 pathways.
J Ethnopharmacol. 2015 Apr 22.
An in vivo and in vitro assessment of the anti-inflammatory, antinociceptive, and immunomodulatory activities of Clematis terniflora DC. extract, participation of aurantiamide acetate.[Pubmed:
25910534]
Clematis terniflora DC. has been widely used as a traditional Chinese medicine for the treatment of tonsillitis, rheumatoid arthritis, and prostatitis. Despite its widespread use in China, there are currently no studies systematically examined its therapeutic effects and mechanism of action. As such, the present study was conducted to evaluate the anti-inflammatory, antinociceptive, and immunomodulatory effects of C. terniflora DC. using rodent and cellular models.
METHODS AND RESULTS:
The anti-inflammatory properties of the 70% ethanol eluted fraction of the 70% ethanol extract of C. terniflora DC. (EECTD) were evaluated using the xylene-induced ear swelling test, the carrageenan-induced edema model, and the cotton pellet granuloma method. Its antinociceptive activities were determined using both the acetic acid-induced writhing test and the hot plate assay. In parallel, we conducted an in vitro assay in LPS-induced RAW264.7 cells to examine the anti-inflammatory effects of EECTD and its purified form, Aurantiamide acetate (AA) on inhibition of nitric oxide (NO) and prostaglandin E2 (PGE2) release. EECTD (300mg/kg) significantly reduced the number of writhing, extended the pain response latency, and suppressed xylene-induced ear swelling. Each EECTD treatment group also had significant inhibition of cotton granulation formation in addition to reduced carrageenan-induced paw edema. EECTD was also shown to alleviate signs of inflammation in histopathological paw sections. However, it had a less noticeable effect on mouse ear swelling in the delayed type hypersensitivity test. A purified compound was isolated from EECTD and its structure was identified as Aurantiamide acetate. In vitro experimental results showed that both EECTD and Aurantiamide acetate were able to significantly inhibit the release of pro-inflammatory cytokines NO and PGE2 on LPS-induced RAW264.7 cells.
CONCLUSIONS:
These results suggest that EECTD has significant anti-inflammatory and antinociceptive activities, partially related to one of the active substances identified as Aurantiamide acetate. We hypothesize that these effects are related to its ability to inhibit the production of cytokines NO and PGE2. However, further work will be needed to determine its exact mechanism of action.
Biosci Biotechnol Biochem. 2001 May;65(5):1195-7.
Aurantiamide acetate, a selective cathepsin inhibitor, produced by Aspergillus penicilloides.[Pubmed:
11440138]
METHODS AND RESULTS:
Aurantiamide acetate was isolated from the fermentation broth of Aspergillus penicilloides for the first time. Aurantiamide acetate inhibited cysteine proteinases, in particular, cathepsin L (3.4.22.15) and B (3.4.22.1) with IC50 of 12 microM and 49 microM, respectively.
In the adjuvant-arthritic rat model, subcutaneously administered 10 mg/kg body weight of this compound suppressed hind paw swellin.
J Cell Mol Med. 2015 May;19(5):1055-64.
Aurantiamide acetate suppresses the growth of malignant gliomas in vitro and in vivo by inhibiting autophagic flux.[Pubmed:
25704599]
We aim to investigate the effect of Aurantiamide acetate isolated from the aerial parts of Clematis terniflora DC against gliomas.
METHODS AND RESULTS:
Human malignant glioma U87 and U251 cells were incubated with different concentrations (0-100 μM) of Aurantiamide acetate. Aurantiamide acetate greatly decreased the cell viability in a dose- and time-dependent manner. It induced moderate mitochondrial fragmentation and the loss of mitochondrial membrane potential. No significant difference was found in the alternation of other intracellular organelles, although F-actin structure was slightly disturbed. Apparent ultrastructure alternation with increased autophagosome and autolysosome accumulation was observed in Aurantiamide acetate-treated cells. The expression of LC3-II was greatly up-regulated in cells exposed to Aurantiamide acetate (P < 0.05 compared with control). The cytoplasmic accumulation of autophagosomes and autolysosomes induced by Aurantiamide acetate treatment was confirmed by fluorescent reporter protein labelling. Administration of chloroquine (CQ), which inhibits the fusion step of autophagosomes, further increased the accumulation of autophagosomes in the cytoplasm of U87 cells. Autophagy inhibition by 3-methyladenine, Bafilomycin A1 or CQ had no influence on Aurantiamide acetate-induced cytotoxicity, whereas autophagy stimulator rapamycin significantly suppressed Aurantiamide acetate-induced cell death. The anti-tumour effects of Aurantiamide acetate were further evaluated in tumour-bearing nude mice. Intratumoural injection of Aurantiamide acetate obviously suppressed tumour growth, and increased number of autophagic vacuoles was observed in tumour tissues of animals receiving Aurantiamide acetate.
CONCLUSIONS:
Our findings suggest that Aurantiamide acetate may suppress the growth of malignant gliomas by blocking autophagic flux.