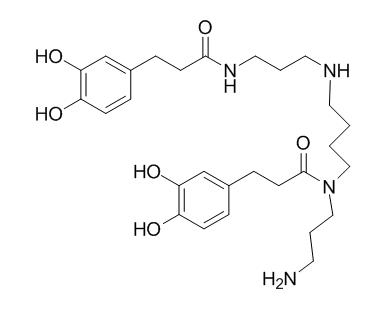
Kukoamine B
Kukoamine B is a potent dual inhibitor for both Lipopolysaccharides (LPS) and oligodeoxynucleotides containing CpG motifs (CpG DNA), LPS and CpG DNA are important pathogenic molecules for the induction of sepsis,are drug targets for sepsis treatment, thus kukoamine B is worthy of further investigation as a potential candidate to treat sepsis. Kukoamine B may potentially serve as an agent for prevention of several human neurodegenerative and other disorders caused by oxidative stress, it has protective effects against hydrogen peroxide (H2O2) induced cell injury and potential mechanisms in SH-SY5Y cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Antimicrob Agents Chemother.2020, AAC.01921-20.
Pharm Biol.2016, 54(7):1255-62
Vet World.2023, 16(3):618-630.
J.of Traditional&Complementary Med.2022, 10.1016:j.jtcme.
Korean Journal of Pharmacognosy2019, 50(4):285-290
Sci Rep.2024, 14(1):28864.
Toxins (Basel).2022, 14(12):824.
J Appl Biol Chem.2024, 67:47,337-343.
Chemistry of Natural Compounds2018, 204-206
Antioxidants (Basel).2023, 12(1):189.
Related and Featured Products
Exp. Ther. Med. ,2015, 9(3):725-32.
A novel role of kukoamine B: Inhibition of the inflammatory response in the livers of lipopolysaccharide-induced septic mice via its unique property of combining with lipopolysaccharide[Pubmed:
25667619 ]
Kukoamine B (KB), derived from the traditional Chinese herb cortex Lycii, exerts anti-inflammatory effects due to its potent affinity with lipopolysaccharide (LPS) and CpG DNA; however, little is known regarding whether the in vivo administration of KB can effectively inhibit inflammation in septic mice.
METHODS AND RESULTS:
The present study thus aimed to investigate the inhibitory effects of KB on the inflammatory response in the livers of LPS-induced septic mice. KB treatment in the LPS-induced septic mice significantly decreased the plasma level of LPS. In addition, KB protected against liver injury, as confirmed by improved histology and decreased aminotransferase levels in the serum. Further experiments revealed that KB attenuated liver myeloperoxidase activity and reduced the expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1. These effects were accompanied by decreases in the levels of tumor necrosis factor α and interleukin-1β in the liver tissue. In parallel, the activity of nuclear factor-κ-gene binding (NF-κB) in the livers of LPS-induced septic mice was markedly inhibited with KB treatment.
CONCLUSIONS:
In combination, these results demonstrate that KB inhibits inflammation in septic mice by reducing the concentrations of plasma LPS, decreasing leukocyte sequestration and interfering with NF-κB activation, and, therefore, suppressing the pro-adhesive phenotype of endothelial cells.
Front Pharmacol . 2019 Jan 22;9:1575.
A Metabolomics Approach to Investigate Kukoamine B-A Potent Natural Product With Anti-diabetic Properties[Pubmed:
30723413.]
Abstract
Due to the surge in type 2 diabetes mellitus (T2DM), treatments for chronic metabolic dysregulations with fewer side-effects are sought. Lycii Cortex (LyC), a traditional Chinese Medicine (TCM) herb has a long history of being widely prescribed to treat T2DM as alternative medicine; however, the bioactive molecules and working mechanism remained unknown. Previous studies revealed Kukoamine B (KB) as a major and featured compound for LyC with bioactivities for anti-oxidation and acute inflammation, which may be related to anti-diabetes properties. This study aims to understand the efficacy and the mode of action of KB in the diabetic (db/db) mouse model using a metabolomics approach. Parallel comparison was conducted using the first-line anti-diabetic drugs, metformin and rosligtazone, as positive controls. The db/db mice were treated with KB (50 mg kg-1 day-1) for 9 weeks. Bodyweight and fasting blood glucose were monitored every 5 and 7 days, respectively. Metabolomics and high-throughput molecular approaches, including lipidomics, targeted metabolomics (Biocrates p180), and cytokine profiling were applied to measure the alteration of serum metabolites and inflammatory biomarkers between different treatments vs. control (db/db mice treated with vehicle). After 9 weeks of treatment, KB lowered blood glucose, without the adverse effects of bodyweight gain and hepatomegaly shown after rosiglitazone treatment. Lipidomics analysis revealed that KB reduced levels of circulating triglycerides, cholesterol, phosphatidylethanolamine, and increased levels of phosphatidylcholines. KB also increased acylcarnitines, and reduced systemic inflammation (cytokine array). Pathway analysis suggested that KB may regulate nuclear transcription factors (e.g., NF-κB and/or PPAR) to reduce inflammation and facilitate a shift toward metabolic and inflammatory homeostasis. Comparison of KB with first-line drugs suggests that rosiglitazone may over-regulate lipid metabolism and anti-inflammatory responses, which may be associated with adverse side effects, while metformin had less impact on lipid and anti-inflammation profiles. Our research from holistic and systemic views supports the conclusion that KB is the bioactive compound of LyC for managing T2DM, and suggests KB as a nutraceutical or a pharmaceutical candidate for T2D treatment. In addition, our research provides insights related to metformin and rosiglitazone action, beyond lowering blood glucose.
Keywords: cytokine array; db/db mouse; Kukoamine B; lipidomics; metabolomics; type 2 diabetes mellitus.
Brit. J. Pharmacol., 2011, 162(6):1274-90.
Kukoamine B, a novel dual inhibitor of LPS and CpG DNA, is a potential candidate for sepsis treatment.[Pubmed:
21108626]
Lipopolysaccharides (LPS) and oligodeoxynucleotides containing CpG motifs (CpG DNA) are important pathogenic molecules for the induction of sepsis, and thus are drug targets for sepsis treatment. The present drugs for treating sepsis act only against either LPS or CpG DNA. Hence, they are not particularly efficient at combating sepsis as the latter two molecules usually cooperate during sepsis. In this study, a natural alkaloid compound Kukoamine B (KB) is presented as a potent dual inhibitor for both LPS and CpG DNA.
METHODS AND RESULTS:
The affinities of KB for LPS and CpG DNA were assessed using biosensor technology. Direct interaction of KB with LPS and CpG DNA were evaluated using neutralization assays. Selective inhibitory activities of KB on pro-inflammatory signal transduction and cytokine expression induced by LPS and CpG DNA were analysed by cellular assays. Protective effects of KB in a sepsis model in mice were elucidated by determining survival and circulatory LPS and tumour necrosis factor-alpha (TNF-α) concentrations.
KB had high affinities for LPS and CpG DNA. It neutralized LPS and CpG DNA and prevented them from interacting with mouse macrophages. KB selectively inhibited LPS- and CpG DNA-induced signal transduction and expression of pro-inflammatory mediators without interfering with signal pathways or cell viability in macrophages. KB protected mice challenged with heat-killed Escherichia coli, and reduced the circulatory levels of LPS and TNF-α.
CONCLUSIONS:
This is the first report of a novel dual inhibitor of LPS and CpG DNA. KB is worthy of further investigation as a potential candidate to treat sepsis.
Environ.Toxicol. Phar. 2015, 40(1):230-40.
Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells.[Pubmed:
26164594 ]
Oxidative stress mediates the cell damage in several neurodegenerative diseases, including multiple sclerosis, Alzheimer's disease (AD) and Parkinson's disease (PD).
METHODS AND RESULTS:
This study aimed at investigating the protective effects of Kukoamine B (KuB) against hydrogen peroxide (H2O2) induced cell injury and potential mechanisms in SH-SY5Y cells. Our results revealed that treatment with KuB prior to H2O2 exposure effectively increased the cell viability, and restored the mitochondria membrane potential (MMP). Furthermore, KuB enhanced the antioxidant enzyme activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) and decreased the malondialdehyde (MDA) content. Moreover, KuB minimized the ROS formation and inhibited mitochondria-apoptotic pathway, MAPKs (p-p38, p-JNK, p-ERK) pathways, but activated PI3K-AKT pathway.
CONCLUSIONS:
In conclusion, we believed that KuB may potentially serve as an agent for prevention of several human neurodegenerative and other disorders caused by oxidative stress.
Columbianetin beta-D-glucopyranoside
Catalog No: CFN95038
CAS No: 55836-35-6
Price: $288/5mg
Rhamnocitrin 3-glucoside
Catalog No: CFN95134
CAS No: 41545-37-3
Price: $318/10mg
(R)-5,3'-Dimethyl hesperidin
Catalog No: CFN95310
CAS No: N/A
Price: $318/5mg
Arvenin III
Catalog No: CFN95327
CAS No: 65597-45-7
Price: $318/5mg
5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No: CFN95376
CAS No: 74064-17-8
Price: $318/10mg
Isochlorogenic acid C 4'-O-glucoside
Catalog No: CFN95405
CAS No: N/A
Price: $413/5mg
Oxytroflavoside E
Catalog No: CFN95464
CAS No: 1391144-84-5
Price: $318/5mg
12beta-Acetoxy-7beta-hydroxy-3,11,15,23-tetraoxo-5alpha-lanosta-8,20-dien-26-oic acid
Catalog No: CFN95515
CAS No: 1245946-62-6
Price: $318/5mg
Odontoside
Catalog No: CFN95530
CAS No: 20300-50-9
Price: $318/10mg
Ephedrannin D1
Catalog No: CFN95547
CAS No: 1592431-55-4
Price: $318/5mg