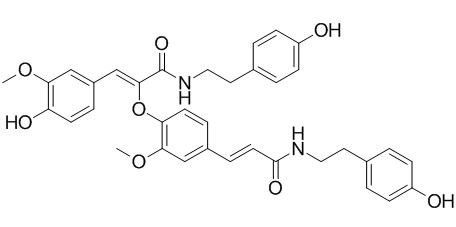
Cannabisin F
Melongenamides B-D and Cannabisin F have anti-inflammatory activity, they exhibit inhibitions of nitric oxide production in lipopolysaccharide-induced RAW 264.7 macrophages with IC50 values ranging from 16.2 to 58.5 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2022, 23(24):16000.
Hanoi University of Pharmacy2023, 14(1):30-39.
PLoS One.2018, 13(4):e0195642
Molecules 2022, 27(3),1047.
J Appl Biol Chem.2024, V67:46-53
Nutrients.2024, 16(14):2267.
J Sep Sci.2023, 46(16):e2300160.
J Am Soc Mass Spectrom.2021, 32(5):1205-1214.
Antimicrob Agents Chemother.2020, AAC.01921-20.
Toxins (Basel).2021, 13(9):593.
Related and Featured Products
Chemical Papers.2014 Mar;68(30):384-391.
Total synthesis of cannabisin F.[Reference:
WebLink]
METHODS AND RESULTS:
A practical eight-step synthesis of lignanamide Cannabisin F starting from vanillin is reported for the first time. This synthetic strategy applies the aldol reaction followed by the Wittig reaction to afford the key 8-O-4′-neolignan intermediate diacid. The diacid was condensed with N,O-protected tyramine giving, after deprotection, Cannabisin F.