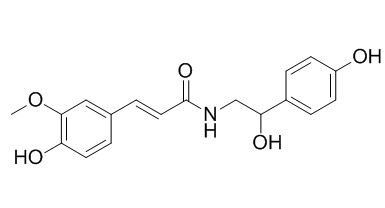
N-Feruloyloctopamine
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biol Pharm Bull.2018, 41(11):1685-1693
Nutrients.2023, 15(12):2810.
Food Analytical Methods2020, 13,1603-1612(2020)
Korean J of Food Science&Technology 2017, 49(2):146-150
Foods2023, 12(23), 4342.
Fitoterapia.2024, 177:106138.
Separations2023, 10(11), 567;
Anticancer Res.2020, 40(10):5529-5538.
Anal Bioanal Chem.2018, 410(5):1561-1569
Jeju National University Graduate School2023, 24478
Related and Featured Products
Bioorg Med Chem Lett . 2017 Feb 15;27(4):989-993.
Inhibition of invasion by N-trans-feruloyloctopamine via AKT, p38MAPK and EMT related signals in hepatocellular carcinoma cells[Pubmed:
28073674]
Abstract
N-trans-feruloyloctopamine (FO) isolated from Garlic skin was identified as the primary antioxidant constituents, however, the effect of which on HCC invasion is still unclear. Herein, the FO was synthesized and its antitumor activities were evaluated in HCC cell lines. Cellular functional analyses have revealed that the reformed FO owns strong abilities of inhibiting cell proliferation and invasion in HCC cells. Molecular data have further showed that FO could significantly decrease the phosphorylation levels of Akt and p38 MAPK. In addition, the expression of Slug was inhibited and the level of E-cadherin increased. Molecular docking analysis indicates that the H-bond and hydrophobic interactions were critical for FO and E-cadherin binding, but FO did not seem to act directly on phosphorylated Akt and p38 MAPK. We have thus concluded that reformed FO inhibits cell invasion might be directly through EMT related signals (E-cadherin) and indirectly through PI3K/Akt, p38 MAPK signaling pathways. FO might be a promising drug in HCC treatment and prognosis.
Keywords: AKT; E-cadherin; Epithelial to mesenchymal transition (EMT); Hepatocellular carcinoma; MAPK; N-trans-feruloyloctopamine (FO).
Plant Cell Physiol. 2005 Mar;46(3):454-66.
Metabolic flux analysis of the phenylpropanoid pathway in elicitor-treated potato tuber tissue.[Pubmed:
15695456]
The effects of beta-1,3-oligosaccharide elicitor on the metabolism of phenylpropanoids in potato tuber were analyzed quantitatively, by monitoring the time-dependent changes in the levels of seven compounds.
METHODS AND RESULTS:
The elicitor treatment caused an increase in the pool size of octopamine and tyramine amides (N-p-coumaroyloctopamine, N-Feruloyloctopamine, N-p-coumaroyltyramine and N-feruloyltyramine), as well as a decrease in that of chlorogenic acid and putrescine amides (caffeoylputrescine and feruloylputrescine). An analysis of metabolic flux using stable isotope labeling and liquid chromatography-spectrometry (LC-MS) detection clearly demonstrated that the changes in the pool size of these compounds were correlated with the changes in their flux for biosynthesis (Jin) upon elicitor treatment. The increase in Jin in the cases of octopamine and tyramine amides was accompanied by an increase in flux for the transformation (Jout), indicating a rapid turnover of these compounds in the elicitor-treated tuber tissue. The result of the flux analysis indicated that the actual activation of the biosynthesis of octopamine and tyramine amides after the elicitor treatment was greater than that estimated from the changes in their levels in the potato tissue.
CONCLUSIONS:
These findings suggest that these amide compounds and their metabolic derivatives play an important role in the defense-related metabolism of phenylpropanoids in potato.