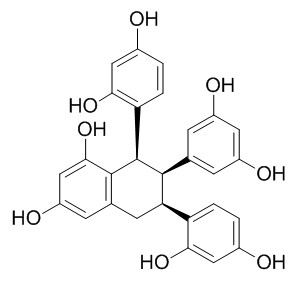
Alboctalol
Alboctalol inhibits mushroom tyrosinase activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep.2017, 7:46299
Molecules.2022, 27(21):7643.
Sci Rep.2024, 14(1):3684.
Chem Biodivers.2023, 20(10):e202300741.
Antioxidants.2022, 11(4), 67.
UDC.2020, 19(4).
J Phys Chem Lett.2021, 12(7):1793-1802.
Trop J Nat Prod Res.2019, 3(1):6-9
Journal of functional foods2018, 171-182
Phytother Res.2022, 10.1002:ptr.7592.
Related and Featured Products
Phytochemistry. 2015 Jul 15;117:424-435.
Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis.[Pubmed:
26188915]
METHODS AND RESULTS:
Twenty-four compounds, including the previously unknown artoxanthocarpuone A, artoxanthocarpuone B, hydroxylakoochin A, methoxylakoochin A, epoxylakoochin A, and artoxanthol, were isolated and characterized spectroscopically. Among them, artoxanthol is stilbene oligomer presumably constructed in a 5,11,12-triphenyl hexahydrochrysene scaffold by a Diels-Alder type of reaction, for which a biosynthetic pathway is proposed. Artoxanthol, Alboctalol, steppogenin, norartocarpetin, resveratrol, oxyresveratrol, and chlorophorin potently inhibited mushroom tyrosinase activity with IC50 values from 0.9 to 5.7μM that were all far stronger than the positive controls. Artoxanthocarpuone A, artoxanthocarpuone B, methoxylakoochin A, lakoochin A, cudraflavone C, artonin A, resveratrol, and chlorophorin reduced tyrosinase activity and inhibited α-melanocyte-stimulating hormone-induced melanin production in B16F10 melanoma cells without affecting cell proliferation.
CONCLUSIONS:
Collectively, the results suggest that the constituents of Artocarpus xanthocarpus have potential to be used as depigmentation agents.
Z Naturforsch C. 2008 Jan-Feb;63(1-2):35-9.
Phenolic constituents from the wood of Morus australis with cytotoxic activity.[Pubmed:
18386485]
METHODS AND RESULTS:
A new methylated flavonol, 5,7,2',4'-tetrahydroxy-3-methoxyflavone (1), had been isolated from the methanol extract of the wood of Morus australis, along with nine known compounds, kuwanon C (2), morusin (3), morachalcone A (4), oxyresveratrol (5), 4'-(2-methyl-2-buten-4-yl)oxyresveratrol (6), moracins M (7) and C (8), Alboctalol (9), and macrourin B (10). The structures of these compounds were determined based on spectral evidence, including UV, IR, NMR, and mass spectra. Cytotoxic properties of compounds 1-10 were evaluated against murine leukemia P-388 cells.
CONCLUSIONS:
The prenylated stilbene 6 and 2-arylbenzofuran 8, and morusin (3) were found to have strong cytotoxic effects with IC50 values of 6.9, 8.7, and 10.1 microM, respectively.
Phytochemistry. 2010 Oct;71(14-15):1708-13.
Secondary metabolites of Bagassa guianensis Aubl. wood: a study of the chemotaxonomy of the Moraceae family.[Pubmed:
20655556]
In order to explain the durability of the Moraceae plant family, phytochemistry of Bagassa guianensis was performed.
METHODS AND RESULTS:
Ethyl acetate extract was obtained from the heartwood and 18 secondary metabolites were isolated, including 6 moracins [6-O-methyl-moracin M, 6-O-methyl-moracin N and moracin Z; previously identified: moracin M, moracin N and moracin P], 8 stilbenoids [presently identified: (-)-epiAlboctalol and arachidin 4; previously identified: Alboctalol, trans-resveratrol, arachidin 2, trans-oxyresveratrol and artogomezianol], 3 previously identified flavonoids, steppogenin, katuranin and dihydromorin, beta-sitosterol and resorcinol. Previous studies suggest that stilbenoids are responsible for the natural durability of wood.
CONCLUSIONS:
Our study has determined that B. guianensis is closely related to Morus sp. in phylogeny and should be included in the Moreae sensu stricto tribe of the Moraceae family.