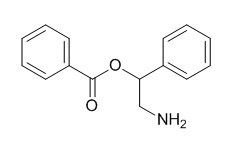
2-Amino-1-phenylethanol
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
ACS Synth Biol.2022, 11(10):3296-3304.
Environ Toxicol.2023, 23929.
Applied Biological Chemistry2023, 66:85.
Neurochem Res.2021, s11064-021-03449-0
Journal of Food and Drug Analysis2023, 31(3), 9.
Pharmaceutical Chemistry Journal2019, 52(12):986-991
Journal of Functional Foods2021, 84:104581
Molecules2022, 27(14),4462
J. of The Korean Society of Food Culture2017, 144-149
J Food Sci.2022, 87(11):4905-4916.
Related and Featured Products
J Phys Chem B. 2007 Aug 23;111(33):9940-54.
The effect of solvation on biomolecular conformation: 2-amino-1-phenylethanol.[Pubmed:
17672488]
Small molecule neurotransmitters form one the most important classes of pharmaceutical molecules. While the behavior of these molecules in their neutral forms in the gas phase is well understood, their behavior in more biologically relevant scenarios (protonated and in aqueous solution) has received comparatively little attention.
METHODS AND RESULTS:
Here we address this problem by using molecular mechanics simulations to build up a detailed picture of the conformational behavior of 2-Amino-1-phenylethanol, a noradrenaline analogue, in aqueous solution in both its neutral and protonated forms. For the sake of comparison, equivalent simulations are also performed on the gas-phase molecules and gas-phase hydrated clusters. These calculations reveal the important role that water has to play in determining the conformational preferences and dynamic behavior of the molecules. Water molecules are found to bridge between the various functional groups within the molecule, significantly affecting their relative stabilities in comparison to the gas-phase values. The reorganization of these solvation structures also provides a mechanism for conformational interconversion.
CONCLUSIONS:
The role of the solvent in mediating interactions between the various functional groups within the molecule suggests that in noradrenaline the catechol groups will be able to interact, albeit indirectly, with the other functional groups, thereby influencing the behavior of the molecule.
J Phys Chem A. 2009 Jul 9;113(27):7769-73.
Shape of biomolecules by free jet microwave spectroscopy: 2-amino-1-phenylethanol and 2-methylamino-1-phenylethanol.[Pubmed:
19505067]
METHODS AND RESULTS:
We report a free jet microwave absorption study of 2-Amino-1-phenylethanol and 2-methylamino-1-phenylethanol, which are analogues of noradrenaline and adrenaline, respectively.
The spectra, recorded under different expansion conditions and with different carrier gases, show the presence of several conformational species: two conformers for 2-Amino-1-phenylethanol and three for 2-methylamino-1-phenylethanol. The assignment is based on the comparison of the experimental rotational constants and the orientation of the molecular dipole moment with the ones predicted by theoretical methods and allows the univocal identification of all observed conformers while intensity measurements give information on their relative stability. All of the observed conformational species are stabilized by an intramolecular hydrogen bond between the hydroxyl hydrogen atom and the amino nitrogen atom, whereas the alkylamino side chain can be in the extended anti or folded gauche conformation.
CONCLUSIONS:
The presence of the bulky methyl group substituent on the alkylamino side chain of 2-methylamino-1-phenylethanol influences the relative stability of the conformers.
Org Biomol Chem. 2008 Feb 7;6(3):458-63.
Solvent control of optical resolution of 2-amino-1-phenylethanol using dehydroabietic acid.[Pubmed:
18219414]
METHODS AND RESULTS:
The optical resolution of 2-Amino-1-phenylethanol (2-APE) by the solvent switch method was investigated using dehydroabietic acid (DAA), a natural chiral acid obtained as one of the main components of disproportionated rosin. The solvent dependency of optical rotation measurements of 2-Amino-1-phenylethanol, DAA and the diastereomeric salts suggested solvent control of optical resolution.
CONCLUSIONS:
Both (R)- and (S)-2-Amino-1-phenylethanol were resolved, as the first success for aminoalcohols, only by changing the resolving solvents: (S)-2-Amino-1-phenylethanol was obtained in high optical purity by a single crystallization operation with polar solvents (epsilon > 50), whereas the efficiency was lower for (R)-2-2-Amino-1-phenylethanol using less polar solvents (20 < epsilon < 40). The results were compared and discussed with reference to the crystal structures of the diastereomeric salts.