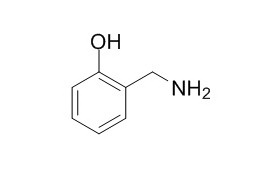
2-Hydroxybenzylamine
2-Hydroxybenzylamine is a novel scavenger that prevents oxidative stress-induced modification of cardiac sodium channels.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2023, 24(4):3682.
Phytother Res.2022, 10.1002:ptr.7626.
Biomolecules.2020, 10(6):925.
Int J Mol Sci.2019, 20(23):E6071
Phytomedicine.2020, 79, 153351
Cell Signal.2022, 99:110433.
Sci Rep. 2018, 1-9
Exp Parasitol.2018, 194:67-78
Processes2021, 9(11),2065.
Molecules.2023, 28(13):4972.
Related and Featured Products
Circulation, 2004 :17-17
2-hydroxybenzylamine: A novel scavenger that prevents oxidative stress-induced modification of cardiac sodium channels[Reference:
WebLink]
METHODS AND RESULTS:
2-Hydroxybenzylamine: A novel scavenger that prevents oxidative stress-induced modification of cardiac sodium channels
Chemistry, 2006,12(11): 3074–3081.
Task-Specific Ionic Liquids Bearing 2-Hydroxybenzylamine Units: Synthesis and Americium-Extraction Studies[Reference:
WebLink]
The synthesis of two task-specific ionic liquids (TSILs) bearing 2-Hydroxybenzylamine entities is described.
METHODS AND RESULTS:
These compounds are based on an imidazolium substructure onto which one hydrobenzylamine-complexing moiety is grafted. We have demonstrated that, whether pure or diluted, TSIL is able to extract americium ions. Furthermore, recovery of americium from the IL phase into a receiving phase can be achieved under acidic conditions.
CONCLUSIONS:
A possible mechanism for the metal-ion partitioning is presented, in which the extraction system is driven by an ion-exchange mechanism.
International Journal of Quantum Chemistry,2010,10(12): 2179–2191.
Impact of the ionic forms on the UV–Vis spectra 2-hydroxybenzylamine. A TD-DFT study[Reference:
WebLink]
METHODS AND RESULTS:
PCM-TD-DFT computations were used to examine the electronic transitions exhibited by the molecular species of 2-Hydroxybenzylamine (2-BNZ).
CONCLUSIONS:
The theoretical results thus obtained were found to accurately fit their experimental counterparts and to afford the assignation of the different experimental electronic transitions to 2-Hydroxybenzylamine tautomers present in the solution.
Also, the HCTH functional was found to accurately reproduce electronic excitations in the cationic species and neutral tautomer, and the B3LYP functional to provide accurate predictions of the transitions for the anionic species and zwitterionic tautomer.