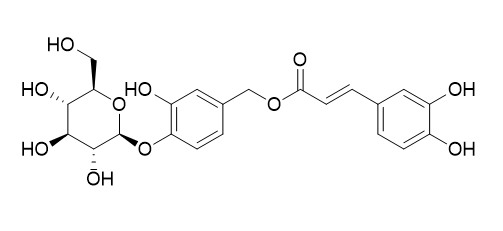
Caffeoylcalleryanin
Caffeoylcalleryanin exerted strong 15-LOX inhibitory activity; IC50 values was 1.59 μM.A. pulchra leaves ethanol extract (EEAPL) affords compounds with antiviral activity, mainly against DENV-2. respectively, while Caffeoylcalleryanin was the most effective anti-DENV-2 constituent, with a SI of 20.0.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem. 2020, 320:126530
National Academy Science Letters2023, s40009.
Chem Biol Interact.2023, 378:110487.
Journal of Functional Foods2022, 99: 105331.
LWT2020, 110397
Biomed Pharmacother.2024, 174:116598.
Molecules.2019, 24(23):E4303
JMicrobiol Biotech Food Sci2021, e4289.
Molecules.2019, 25(1):E103
Talanta.2022, 249:123645.
Related and Featured Products
Molecules . 2013 Aug 16;18(8):9919-9932.
Chemistry and Antiviral Activity of Arrabidaea pulchra (Bignoniaceae)[Pubmed:
23959197]
The aim of the present work was to carry out a bioguided isolation of antiviral chemical constituents from an ethanol extract of leaves from Arrabidaea pulchra (Cham.) Sandwith (EEAPL) that had shown in vitro activity in a previous screening using DNA and RNA viruses. The activity of EEPAL was evaluated against the DNA viruses Human herpesvirus 1 (HSV-1) and Vaccinia virus Western Reserve (VACV-WR) as well as against the RNA viruses Murine encephalomyocarditis virus (EMCV), and Dengue virus 2 (DENV-2) by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Cytotoxicity was determined in LLCMK2 and Vero cells and the Selectivity Indexes (SI) were calculated. The most potent effect was observed against DENV-2 (EC50 46.8 ± 1.6 μg mL(-1); SI 2.7). For HSV-1 and VACV-WR EC50 values > 200 μg mL(-1) were determined, while no inhibition of the cytopathic effect was observed with EMCV. Bioguided fractionation of EEAPL by partition between immiscible solvents followed by chromatography over a Sephadex LH20 column afforded two arylpropanoid glycosides, verbascoside (AP 1) and Caffeoylcalleryanin (AP 2), along with a terpenoid, ursolic acid (AP 3). AP 1 and AP 3 exhibited similar anti-DENV-2 profiles, with SI values of 3.8 and 3.1, respectively, while AP 2 was the most effective anti-DENV-2 constituent, with a SI of 20.0. Our results show that A. pulchra leaves ethanol extract (EEAPL) affords compounds with antiviral activity, mainly against DENV-2.
Nat Prod Res . 2015;29(11):1083-1086.
Lipoxygenase inhibitory activity of Cuspidaria pulchra and isolated compounds[Pubmed:
25428032]
This work evaluated the in vitro inhibitory activity of the crude ethanolic extract from the aerial parts of Cuspidaria pulchra (Cham.) L.G. Lohmann against 15-lipoxygenase (15-LOX). The bioassay-guided fractionation of the n-butanol fraction, which displayed the highest activity, led to the isolation of three compounds: Caffeoylcalleryanin (1), verbascoside (2) and 6-hydroxyluteolin-7-O-β-glucoside (3). Assessment of the ability of the isolated compounds to inhibit 15-LOX revealed that compounds 1, 2 and 3 exerted strong 15-LOX inhibitory activity; IC50 values were 1.59, 1.76 and 2.35 μM respectively. The XTT assay showed that none of the isolated compounds seemed to be significantly toxic.