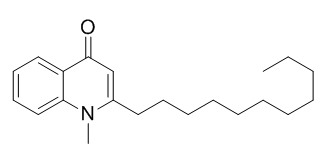
1-Methyl-2-undecylquinolin-4(1H)-one
1-Methyl-2-undecyl-4(1H)-quinolone, and dihydroevocarpine should also be served as the chemical markers together with evodiamine for the quality control of Evodia rutaecarpa (Juss.) Benth. 1-Methyl-2-undecyl-4(1H)-quinolone shows a selective inhibition of type B MAO (MAO-B) activity with the IC(50) value of 15.3 microM using a substrate kynuramine, but does not inhibit type A MAO (MAO-A) activity.It can mitigate high phosphate-induced human aortic valve interstitial cells (HAVICs) calcification by inhibiting phosphate cotransporter (PiT-1) gene expression. It also shows moderate antiangiogenic activity against human tumor cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Fitoterapia.2024, 106006.
Int J Mol Sci.2022, 23(15):8687.
Chem Biol Interact.2019, 298:1-7
Molecules.2019, 25(1):E103
Anticancer Res.2018, 38(4):2127-2135
Eur J Pharmacol.2022, 917:174744.
Chemistry of Natural Compounds2019, 55(1):127-130
Metabolites.2023, 13(6):689.
Sci Rep.2017, 7:467-479
Molecules.2019, 24(4):E709
Related and Featured Products
J Pharmacol Sci. 2016 May;131(1):51-7
1-Methyl-2-undecyl-4(1H)-quinolone, a derivative of quinolone alkaloid evocarpine, attenuates high phosphate-induced calcification of human aortic valve interstitial cells by inhibiting phosphate cotransporter PiT-1.[Pubmed:
27165707 ]
An abnormally high serum phosphate level induces calcific aortic stenosis (CAS), which is characterized by ectopic valve calcification and stenosis of the orifice area. Inhibition of ectopic calcification is a critical function of any internal medical therapy for CAS disease.
The aim of the present study was to investigate the inhibitory effects of several derivatives of evocarpine, methanolic extracts from the fruits of Evodia rutaecarpa Bentham (Japanese name: Go-Shu-Yu) on the high phosphate-induced calcification of human aortic valve interstitial cells (HAVICs) obtained from patients with CAS.
METHODS AND RESULTS:
High phosphate (3.2 mM) concentrations significantly increased the calcification of HAVICs after 7 days of culture. This calcification was completely inhibited in the presence of sodium phosphonoformate (PFA), a selective inhibitor of the type III sodium-dependent phosphate cotransporter (PiT-1). PiT-1 contributes to phosphate uptake, resulting in calcification. 1-Methyl-2-undecyl-4(1H)-quinolone (1-Methyl-2-undecylquinolin-4(1H)-one,MUQ; 30-300 nM), but not evocarpine or its derivatives dihydroevocarpine and 1-methyl-2-nonyl-4(1H)-quinolone, inhibited the high phosphate-induced HAVICs calcification in a concentration-dependent manner. Although all of the evocarpine derivatives attenuated alkaline phosphatase activity, only MUQ also decreased PiT-1 gene expression with cellular PiT-1 protein diminution.
CONCLUSIONS:
These results suggest that MUQ mitigated high phosphate-induced HAVICs calcification by inhibiting PiT-1 gene expression.
J Anal Methods Chem. 2013;2013:827361.
Simultaneous Quantification of Limonin, Two Indolequinazoline Alkaloids, and Four Quinolone Alkaloids in Evodia rutaecarpa (Juss.) Benth by HPLC-DAD Method.[Pubmed:
23738236 ]
METHODS AND RESULTS:
A simple and efficient HPLC-DAD (225 nm) method was developed and validated for the simultaneous determination of limonin and six key alkaloids (evodiamine, rutaecarpine, 1-methyl-2-undecyl-4(1H)-quinolone(1-Methyl-2-undecylquinolin-4(1H)-one), evocarpine, 1-methy-2-[(6Z,9Z)]-6,9-pentadecadienyl-4-(1H)-quinolone, and dihydroevocarpine) in Evodia rutaecarpa (Juss.) Benth, which has been widely used as one of the Traditional Chinese Medicines.
CONCLUSIONS:
This study indicated that the quality control of E. rutaecarpa could be simplified to the measurement of four constituents, and that limonin, 1-methyl-2-undecyl-4(1H)-quinolone(1-Methyl-2-undecylquinolin-4(1H)-one), and dihydroevocarpine should also be served as the chemical markers together with evodiamine for the quality control of Evodia rutaecarpa (Juss.) Benth.
Planta Medica,2012,78(11):1202-3.
Studies on the chemical constituents of Evodia rutaecarpa and Evodia rutaecarpa passed with Liquorice and biological activities[Reference:
WebLink]
METHODS AND RESULTS:
17 indole quinoline alkaloids, 8 limonins, 7 flavonoids, 7 triterpenoids and 11 other compounds were obtained from E. rutaecarpa and E. rutaecarpa passed with Liquorice. 6 of them are new compounds. About 19 compounds were tested by replanting tumor to mice, angioma of zebrafish model for antiangiogenic drug screening and human tumor cells in vitro.
CONCLUSIONS:
Thus compounds evodiagenine (2), evodialimonin (3), rhetsinine (4), dehydroevodiamine (5), wuchuyuamide I (7) and 1-methyl-2-undecyl-4(1H)-quinolone (1-Methyl-2-undecylquinolin-4(1H)-one,8) showed moderate activity against human tumor cells, dievodiamine (1) and evodiamine (6) showed good activity. The inhibitory rate of 4 on sarcoma S180 was 57.78% at the dose of 5–10mg/kg·d in vivo. Moreover, the inhibition of the active selectivity of cycloxygenase 1 and 4 showed a stronger effect.
Chem Pharm Bull (Tokyo). 2003 Apr;51(4):409-11.
1-methyl-2-undecyl-4(1H)-quinolone as an irreversible and selective inhibitor of type B monoamine oxidase.[Pubmed:
12672993]
METHODS AND RESULTS:
The inhibitory compound of monoamine oxidase (MAO) activity was isolated from the CH(2)Cl(2) fraction of the fructus of Evodia rutaecarpa and identified as 1-methyl-2-undecyl-4(1H)-quinolone (1-Methyl-2-undecylquinolin-4(1H)-one,1). Compound 1 showed a selective inhibition of type B MAO (MAO-B) activity with the IC(50) value of 15.3 microM using a substrate kynuramine, but did not inhibit type A MAO (MAO-A) activity. The kinetic analysis using Lineweaver-Burk plots indicated that compound 1 competitively inhibited MAO-B activity with the K(i) value of 9.91 microM. The inhibition of MAO-B by compound 1 was found to be irreversible by dialysis of the incubation mixture.
CONCLUSIONS:
These results suggest that compound 1 is a potent irreversible inhibitor of MAO-B, and may regulate catecholamine content in the neurons.