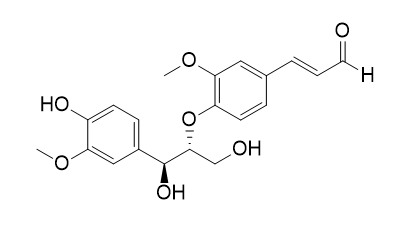
Erythro-Guaiacylglycerol-beta-coniferyl aldehyde ether
Erythro-Guaiacylglycerol-beta-coniferyl aldehyde ether,cytotoxic effects on human cancer cell lines
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep.2024, 14(1):3684.
Phytomedicine.2019, 62:152962
Journal of Apicultural Research2021, 60(1).
Molecules.2019, 24(9):E1719
Toxicological Research2020, doi: 10.1007.
Food Chem X.2024, 24:101794.
Dermatologica Sinica2024, 42(1):p19-30.
Journal of Holistic Integrative Pharmacy2024, 5(1):45-55.
J Ethnopharmacol.2017, 206:327-336
Evid Based Complement Alternat Med.2015, 2015:165457
Related and Featured Products
Bioorg Chem . 2018 Dec;81:382-388
Stereoisomeric guaiacylglycerol-β-coniferyl aldehyde ether induces distinctive apoptosis by downregulation of MEK/ERK pathway in hepatocellular carcinoma cells[Pubmed:
30196208]
Two 8-O-4'-type neolignan epimers erythro-guaiacylglycerol-β-coniferyl aldehyde ether (1) and threo-guaiacylglycerol-β-coniferyl aldehyde ether (2) were isolated from the stems of Picrasma quassioides. Further chiral separation gave two pairs of enantiomers 1a/1b and 2a/2b. The cytotoxicity assay against hepatocellular carcinoma Hep3B and HepG2 cells was evaluated by MTT assay. The results showed that 1b (IC50 = 45.56 μM) and 2b (IC50 = 39.02 μM) had more cytotoxic effect than its enantiomers 1a (IC50 = 82.66 μM) and 2a (IC50 = 67.97 μM) in Hep3B cells, respectively. Moreover, 1b and 2b could induce more apoptotic cells as well as higher reactive oxygen species (ROS) generation than 1a and 2a at 50 μM. In addition, a further study on the phosphoinositide 3-kinase (PI3K)/AKT and mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathways was investigated. The results revealed that all compounds had no significant effect on PI3K/AKT pathway, however, 1b and 2b attenuated the relative levels of p-MEK and p-ERK when compared with 1a and 2a. Taken together, the absolute configurations of guaiacylglycerol-β-coniferyl aldehyde ether had an impact on the inhibitory effect on Hep3B cells. The inactivation of MEK/ERK signaling pathway might contribute to apoptosis induction and ROS generation in 1b- and 2b-treated cells.
Arch Pharm Res . 2007 Apr;30(4):402-407
Lignans from the fruits of Cornus kousa Burg. and their cytotoxic effects on human cancer cell lines[Pubmed:
17489353]
The fruits of Cornus kousa Burg. were extracted with 80% aqueous MeOH, and the concentrated extract partitioned with EtOAc, n-BuOH and H2O. Six lignans were isolated from the EtOAc fraction through repeated silica gel, ODS and Sephadex LH-20 column chromatographies. From the physico-chemical data, including NMR, MS and IR, the chemical structures of the compounds were determined to be (+)-pinoresinol (1), (-)-balanophonin (2), (+)-laricresinol (3), Erythro-Guaiacylglycerol-beta-coniferyl aldehyde ether (4), threo-guaiacylglycerol-beta-coniferyl aldehyde ether (5) and dihydrodehydrodiconiferyl alcohol (6), which were isolated for the first time from this plant. Most of these compounds showed cytotoxicity against human colon carcinoma (HCT-116) and human hepatocellular carcinoma (HepG2) cell lines in vitro, with IC50 values ranging from 19.1 to 71.3 microg/mL.