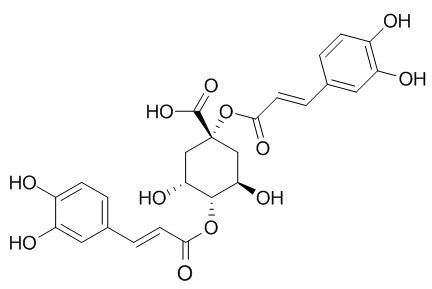
1,4-Dicaffeoylquinic acid
1,4-Dicaffeoylquinic acid is a potent and highly selective class of HIV-1 integrase inhibitors, inhibitsHIV-1 replication in MT-2 cell culture at non-toxic concentrations.1,4-Dicaffeoylquinic acid also has antioxidant activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules2022, 27(11):3606.
Analytical sci. & Tech2016, 186-193
J Pharmaceutical and Biomedical Analysis2022, 114631.
Int J Mol Sci.2022, 23(23):15213.
Int J Nanomedicine.2024, 19:1683-1697.
Life (Basel).2022, 12(12):2107.
Am J Chin Med.2016, 44(6):1255-1271
Nat Commun.2023, 14(1):5075.
Heliyon.2024, 10(7):e28364.
Drug Des Devel Ther.2020, 14:969-976.
Related and Featured Products
Pharmacogn Mag . 2015 Oct;11(Suppl 4):S585-91.
Phytochemical Analysis on Quantification and the Inhibitory Effects on Inflammatory Responses from the Fruit of Xanthii fructus[Pubmed:
27013799]
Abstract
Objective: Xanthii fructus (Compositae) is a traditional herbal medicine used for treating headache, toothache, pruritus, empyema, and rhinitis. In this study of the quality control of X. fructus, we performed simultaneous analysis of nine marker compounds: Protocatechuic acid (1), chlorogenic acid (2), caffeic acid (3), 4,5-dicaffeoylquinic acid (4), ferulic acid (5), 3,5-dicaffeoylquinic acid (6), 1,3-dicaffeoylquinic acid (7), 1,4-Dicaffeoylquinic acid (8), and 4,5-dicaffeoylquinic acid (9).
Materials and methods: Nine components were separated using reversed-phase SunFire™ C18 analytical column and analyzed using high-performance liquid chromatography. We examined the biological effects of the nine marker compounds by determining their anti-inflammatory activities in the murine macrophage cell line RAW 264.7.
Results: Among the nine marker compounds, eight significantly inhibited lipopolysaccharide (LPS)-stimulated tumor necrosis factor-alpha (TNF-α) production. 1, 3, 5 had significant inhibitory effects on LPS-induced prostaglandin E2 (PGE2) production in RAW 264.7 cells. None of the tested marker compounds had a significant effect on interleukin-6 production in LPS-treated RAW 264.7 cells. Our data demonstrated that each marker compound from X. fructus exerts anti-inflammatory activity by targeting different inflammation-related pathways such as the TNF-α or PGE2 pathway.
Conclusion: Further experiments using in vitro and in vivo models are needed to identify the mechanisms responsible for the anti-inflammatory properties of each marker compound.
Summary: Simultaneous analysis of nine phenylpropanoids in the Xanthii fructus was established using HPLC-PDA system.1,4-Dicaffeoylquinic acid significantly inhibited LPS-stimulated TNF-a production.Protocatechuic acid, caffeic acid and ferulic acid had significant inhibitory effects on LPS-induced PGE2 production in RAW 264.7 cells.
Keywords: Anti-inflammatory effect; RAW 264.7; Xanthii fructus; herbal medicine; high-performance liquid chromatography.
Chin J Nat Med. 2014 Feb;12(2):131-8.
Inhibitory effect of medicinal plant-derived carboxylic acids on the human transporters hOAT1, hOAT3, hOATP1B1, and hOATP2B1.[Pubmed:
24636064]
In this study, uptake experiments were performed to assess the inhibitory effects of cinnamic acid, ferulic acid, oleanolic acid, deoxycholic acid, and cynarin(1,4-Dicaffeoylquinic acid ) on hOAT1, hOAT3, hOATP1B1, and hOATP2B1.
METHODS AND RESULTS:
After a drug-drug interaction (DDI) investigation, cinnamic acid, ferulic acid, deoxycholic acid, and cynarin(1,4-Dicaffeoylquinic acid ) were found and validated to inhibit hOAT1 in a competitive manner, and deoxycholic acid was found to be an inhibitor of all four transporters. The apparent 50% inhibitory concentrations of cinnamic acid, ferulic acid, deoxycholic acid, and cynarin(1,4-Dicaffeoylquinic acid ) were estimated to be 133.87, 3.69, 90.03 and 6.03 μmol·L(-1) for hOAT1, respectively. The apparent 50% inhibitory concentrations of deoxycholic acid were estimated to be 9.57 μmol·L(-1) for hOAT3, 70.54 μmol·L(-1) for hOATP1B1, and 168.27 μmol·L(-1) for hOATP2B1. Because cinnamic acid, ferulic acid, and cynarin are ingredients of food or food additives, the present study suggests there are new food-drug interactions to be disclosed.