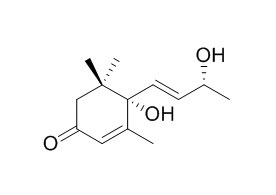
Vomifoliol
Vomifoliol is most likely the immediate precursor of dehydrovomifoliol, because of the organism converted abscisic acid (ABA), to dehydrovomifoliol as the major metabolise, and a cell-free extract exhibits vomifoliol dehydrogenase activity. Vomifoliol and stigmasterol have anti-proliferative activity on human lung cancer cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Cosmet Sci.2019, 41(1):12-20
Molecules.2019, 24(19):E3417
Sci Rep.2019, 9(1):18080
FUTURE VIROLOGYVOL.2023, 18(5).
Evid Based Complement Alternat Med.2017, 2017:1401279
BMC Complement Med Ther. 2020, 20(1):94.
The Journal of Korean Medicine2022, 43(3): 79-93.
Academic J of Second Military Medical University2018, 39(11)
Int J Mol Sci.2024, 25(17):9730.
BioResources J.2020, 15(3).
Related and Featured Products
Molecules. 2010 Apr 22;15(4):2911-24.
Organic extractives from Mentha spp. honey and the bee-stomach: methyl syringate, vomifoliol, terpenediol I, hotrienol and other compounds.[Pubmed:
20428087]
METHODS AND RESULTS:
The GC and GC/MS analyses of the solvent organic extractive from the stomach of the bees, having collected Mentha spp. nectar, revealed the presence of methyl syringate (6.6%), terpendiol I (5.0%) and Vomifoliol (3.0%) that can be attributed to the plant origin. Other major compounds from the bee-stomach were related to the composition of cuticular waxes and less to pheromones. Organic extractives from Mentha spp. honey were obtained by solvent-free headspace solid-phase microextraction (HS-SPME) and ultrasonic solvent extraction (USE) and analyzed by GC and GC/MS. The major honey headspace compounds were hotrienol (31.1%-38.5%), 2-methoxy-4-methylphenol (0.5-6.0%), cis- and trans-linalool oxides (0.9-2.8%), linalool (1.0-3.1%) and neroloxide (0.9-1.9%). Methyl syringate was the most abundant compound (38.3-56.2%) in the honey solvent extractives followed by Vomifoliol (7.0-26.6%).
CONCLUSIONS:
Comparison of the honey organic extractives with the corresponding bee-stomach extractive indicated that methyl syringate and vomofoliol were transferred to the honey while terpendiol I was partially transformed to hotrienol in ripened honey.
Phytochemistry, 1984, 23(12):2769-71.
Metabolism of abscisic acid: Bacterial conversion to dehydrovomifoliol and vomifoliol dehydrogenase activity.[Reference:
WebLink]
METHODS AND RESULTS:
A species of Corynebacterium, capable of metabolizing abscisic acid (ABA), was isolated from soil. The organism converted ABA to dehydroVomifoliol [(±)-1′-hydroxy-4′-keto-α-ionone] as the major metabolise. A cell-free extract exhibited Vomifoliol dehydrogenase activity.
CONCLUSIONS:
This suggests that Vomifoliol is most likely the immediate precursor of dehydroVomifoliol.
Molecules. 2011 Mar 16;16(3):2507-18.
Screening of natural organic volatiles from Prunus mahaleb L. honey: coumarin and vomifoliol as nonspecific biomarkers.[Pubmed:
21407151]
METHODS AND RESULTS:
Headspace solid-phase microextraction (HS-SPME; PDMS/DVB fibre) and ultrasonic solvent extraction (USE; solvent A: pentane and diethyl ether (1:2 v/v), solvent B: dichloromethane) followed by gas chromatography and mass spectrometry (GC, GC-MS) were used for the analysis of Prunus mahaleb L. honey samples.
Screening was focused toward chemical composition of natural organic volatiles to determine if it is useful as a method of determining honey-sourcing. A total of 34 compounds were identified in the headspace and 49 in the extracts that included terpenes, norisoprenoids and benzene derivatives, followed by minor percentages of aliphatic compounds and furan derivatives.
CONCLUSIONS:
High Vomifoliol percentages (10.7%-24.2%) in both extracts (dominant in solvent B) and coumarin (0.3%-2.4%) from the extracts (more abundant in solvent A) and headspace (0.9%-1.8%) were considered characteristic for P. mahaleb honey and highlighted as potential nonspecific biomarkers of the honey's botanical origin. In addition, comparison with P. mahaleb flowers, leaves, bark and wood volatiles from our previous research revealed common compounds among norisoprenoids and benzene derivatives.