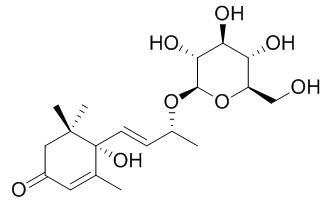
Roseoside
Roseoside possesses strong antioxidant properties, inhibiting lipid oxidation by 86.4, at 50 microg/mL. Roseoside has insulinotropic activity, enhances insulin release from the β-cell line INS-1.Roseoside can significantly delay carcinogenesis induced by peroxynitrite (initiator) and TPA (promoter). (6S,9R)-Roseoside also can inhibit the histamine release from rat peritoneal exudate cells induced by antigen-antibody reaction.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Babol University of Medical Sciences2024, rs-4289336
Integr Med Res.2024, 13(1):101025.
Front Plant Sci.2023, 14:1207940.
Heliyon.2024, 10(7):e28364.
Food Chem.2018, 262:78-85
Processes2021, 9(5),831.
Molecules.2023, 28(13):4971.
Agronomy2020, 10(10),1489
Univerzita Karlova2021, 20.500.11956.
Environ Toxicol.2023, 38(5):1174-1184.
Related and Featured Products
Planta Med. 2010 Jul;76(10):995-7.
Enhancement of insulin release from the beta-cell line INS-1 by an ethanolic extract of Bauhinia variegata and its major constituent roseoside.[Pubmed:
20143296]
Plants of the genus Bauhinia are used in several countries worldwide for the treatment of diabetes, and several related species have been shown to have hypoglycaemic effects in vivo in both normoglycaemic and alloxan- and streptozotocin-treated animal models.
METHODS AND RESULTS:
In this study, the insulin-secreting cell line INS-1 was used to examine the effects of the crude ethanolic extract of leaves of B. variegata L. var. Candida Voidt and its major metabolite (6 S,7 E,9 R)-9-hydroxymegastigma-4,7-dien-3-one-9- beta-glycopyraroside (Roseoside) on insulinotropic activity.
CONCLUSIONS:
The crude extracts and the major metabolite were shown to increase insulin secretion in a dose-dependant manner.
J Agric Food Chem. 2002 Apr 10;50(8):2400-3.
Antitumor activity of compounds isolated from leaves of Eriobotrya japonica.[Pubmed:
11929303]
METHODS AND RESULTS:
In a search for possible antitumor agents from natural sources, megastigmane glycosides and polyphenolic constituents isolated from the leaves of Eriobotrya japonica (Rosaceae) were found to inhibit the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced activation of Epstein-Barr virus early antigen in Raji cells. Roseoside and procyanidin B-2 were among the active compounds found in an in vitro assay; these compounds were further assessed for antitumor activity in vivo in a two-stage carcinogenesis assay on mouse skin.
CONCLUSIONS:
Roseoside significantly delayed carcinogenesis induced by peroxynitrite (initiator) and TPA (promoter), and its potency was comparable to that of a green tea polyphenol, (-)-epigallocatechin 3-O-gallate, in the same assay.
Life Sci. 2004 Jun 25;75(6):753-63.
Lipid peroxidation inhibitory compounds from daylily (Hemerocallis fulva) leaves.[Pubmed:
15172183 ]
Daylilies (Hemerocallis spp.) have been used as food and in traditional medicine for thousands of years in eastern Asia.
METHODS AND RESULTS:
The leaves of the plant are used in the treatment of inflammation and jaundice. In studies of the aqueous methanol extracts of fresh Hemerocallis fulva leaves, 1',2',3',4'-tetrahydro-5'-deoxy-pinnatanine (1), pinnatanine (2), Roseoside (3), phlomuroside (4), lariciresinol (5), adenosine (6), quercetin 3-O-beta-D-glucoside (7), quercetin 3,7-O-beta-D-diglucopyranoside (8), quercetin 3-O-alpha-L-rhamnopyransol-(1-->6)-beta-D-glucopyranosol-7-O-beta-D-glucopyranoside (9), isorhamnetin-3-O-beta-D-6'-acetylglucopyranoside (10) and isorhamnetin-3-O-beta-D-6'-acetylgalactopyranoside (11) were isolated. All of these compounds were tested for their in vitro lipid peroxidation inhibitory activities.
CONCLUSIONS:
Compounds 3-5 and 7-11 were found to possess strong antioxidant properties, inhibiting lipid oxidation by 86.4, 72.7, 90.1, 79.7, 82.4, 89.3, 82.2, and 93.2%, respectively at 50 microg/mL. Compound 1 is novel and compounds 3-6 and 8-11 described here in are isolated for the first time from daylily leaves.
Chem Pharm Bull (Tokyo). 1997 Mar;45(3):464-9.
Medicinal foodstuffs. V. Moroheiya. (1): Absolute stereostructures of corchoionosides A, B, and C, histamine release inhibitors from the leaves of Vietnamese Corchorus olitorius L. (Tiliaceae).[Pubmed:
9085554]
METHODS AND RESULTS:
Three new ionone glucosides named corchoionosides A, B, and C were isolated from the leaves of Corchorus olitorius, commonly called "moroheiya" in Japanese, together with seven known compounds, an ionone glucoside (6S,9R)-Roseoside, a monoterpene glucoside betulalbuside A, two flavonol glucosides astragalin and isoquercitrin, two coumarin glucosides scopolin and cichoriine, and chlorogenic acid. The absolute stereostructures of corchoionosides A, B, and C were determined by chemical and physiochemical evidence, which included the result of application of a modified Mosher's method, the CD helicity rule, and chemical correlation with (6S,9R)-Roseoside.
CONCLUSIONS:
Corchoionosides A and B and (6S,9R)-Roseoside were found to inhibit the histamine release from rat peritoneal exudate cells induced by antigen-antibody reaction.
Bioorg Med Chem. 2009 Jan 1;17(1):189-94.
A simple synthesis of four stereoisomers of roseoside and their inhibitory activity on leukotriene release from mice bone marrow-derived cultured mast cells.[Pubmed:
19026550 ]
METHODS AND RESULTS:
Four stereoisomers of Roseoside (vomifoliol glucosides) were synthesized using glucose as a chiral resolving reagent. The four synthetic stereoisomers exhibited inhibitory activity on leukotriene release from mouse bone marrow-derived cultured mast cells (BMCMC).
CONCLUSIONS:
The (6S)-isomers of Roseoside were about twice as active as (6R)-isomers.