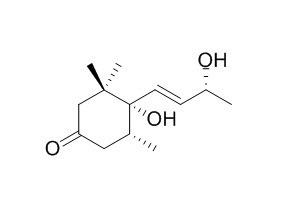
4,5-Dihydroblumenol A
4, 5-Dihydroblumenol shows significant inhibition against HepG2 cells transected with cloned hepatitis B virus DNA.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
The Japan Society for Analy. Chem.2017, 66(8):613-617
Fundam. Toxicol. Sci.2024, 11(4):197-204
Food Chem.2019, 276:768-775
Appl. Sci.2024, 14(12), 5280.
Molecules.2024, 29(6):1392.
Vet World.2023, 16(3):618-630.
Comp. & Mathematical Methods in Med.2022, 5475559.
Appl Microbiol Biotechnol.2024, 108(1):207.
Korean J of Crop Science2019, 452-458
Metabolites2022, 12(6),507.
Related and Featured Products
Molecules. 2008 Jun 1;13(6):1219-29.
Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity.[Pubmed:
18596648]
Dragon's blood (Sangre de drago), a viscous red sap derived from Croton lechleri Muell-Arg (Euphorbiaceae), is extensively used by indigenous cultures of the Amazonian basin for its wound healing properties. The aim of this study was to identify the minor secondary metabolites and test the antioxidant activity of this sustance.
METHODS AND RESULTS:
A bioguided fractionation of the n-hexane, chloroform, n-butanol, and aqueous extracts led to the isolation of 15 compounds: three megastigmanes, four flavan-3-ols, three phenylpropanoids, three lignans, a clerodane, and the alkaloid taspine. In addition to these known molecules, six compounds were isolated and identified for the first time in the latex: blumenol B, blumenol C, 4,5-Dihydroblumenol A, erythro-guaiacyl-glyceryl-beta-O-4'- dihydroconiferyl ether, 2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-propane-1,3-diol and floribundic acid glucoside. Combinations of spectroscopic methods ((1)H-, (13)C- NMR and 2D-NMR experiments), ESI-MS, and literature comparisons were used for compound identification.
In vitro antioxidant activities were assessed by DPPH, total antioxidant capacity and lipid peroxidation assays.
CONCLUSIONS:
Flavan-3-ols derivatives (as major phenolic compounds in the latex) exhibited the highest antioxidant activity.
Chinese Traditional & Herbal Drugs, 2007, 38(7): 976-9.
Antivirus constituents from Alternanthera philoxeroides.[Reference:
WebLink]
To investigate the anti-HBV constituents from Alternanthera philoxeroides.
METHODS AND RESULTS:
The constituents were isolated with silica gel and gel permeation chromatography,and purified by HPLC.Their structures were elucidated by spectroscopy.The antivirus effects of the isolated compounds were tested by ELISA method in vitro. Ten compounds were isolated and elucidated as followings:oleanolic acid(Ⅰ),oleanolic acid 3-O-β-D-glucuronopyranoside(Ⅱ),oleanolic acid 28-O-β-D-glucopyranoside(Ⅲ),chikusetsusaponin Ⅳ a methyl ester(Ⅳ),4,5-dihydroblumenol(Ⅴ),N-trans-feruloyl 3-methyldopamine(Ⅵ),N-trans-feruloyl tyramine(Ⅶ),3β-hydroxystigmast-5-en-7-one(Ⅷ),24-methylenecycloartanol(Ⅸ),and cycloeucalenol(Ⅹ).The values of inhibition percent of compounds Ⅰ-Ⅲ,Ⅴ-Ⅶ revealed a significant distinction compared to the control group.Compounds Ⅱ and Ⅴ showed significant inhibition against HepG2 cells transected with cloned hepatitis B virus DNA,their inhibitive ratios were 85.38% and 87.37% at 50 μg/mL,respectively.
CONCLUSIONS:
Compounds Ⅳ-Ⅶ are isolated from this plant for the first time and phenolic amides have been determined as the new structure type from the plants of Alternanthera Forsk.Compounds Ⅱ and Ⅴ from A.philoxeroides show the more significant anti-HBV activities.
Zhongguo Zhong Yao Za Zhi. 2013 Jul;38(14):2321-4.
[Chemical constituents from stems of Brucea mollis and their cytotoxic activity].[Pubmed:
24199564]
METHODS AND RESULTS:
Ten compounds were isolated from the stems of Brucea mollis by various chromatographic techniques such as column chromatography on silica gel and Sephadex LH-20, and preparative HPLC, and their structures were elucidated as deacetylated isobrucein B (1), indaquassin X (2), cleomiscosin A (3), cleomiscosin B (4), (+)-lyoniresinol (5), (+)-epipinoresinol(6), (+)-pinoresinol (7), (+)-syringaresinol (8), 4,5-Dihydroblumenol A (9) and adenosine (10) on the basis of spectroscopic data analysiS.
CONCLUSIONS:
All compounds were obtained from this plant for the first time, moreover, compound 1 was a new natural product. Compound 2 showed significant cytotoxic activities against the human cell lines HT-29, HepG2, BGC-823 and SKOV3 with IC50 values of 0.84-3.97 micromol x L(-1).
Dactylorhin A
Catalog No: CFN95032
CAS No: 256459-34-4
Price: $238/20mg
Poricoic acid G
Catalog No: CFN95056
CAS No: 415724-84-4
Price: $318/5mg
Daidzein-4'-glucoside
Catalog No: CFN95142
CAS No: 58970-69-7
Price: $318/5mg
Lappaol B
Catalog No: CFN95242
CAS No: 62359-60-8
Price: $333/10mg
(3beta,22alpha)-26-(beta-glucopyranosyloxy)-22-methoxyfurost-5-en-3-yl 2-O-(6-deoxy-alpha-mannopyranosyl)-beta-glucopyranosiduronic acid
Catalog No: CFN95321
CAS No: 107783-53-9
Price: $318/20mg
Toddalolactone 3'-O-methyl ether (6-(2-Hydroxy-3-methoxy-3-methylbutyl)-5,7-dimethoxycoumarin)
Catalog No: CFN95372
CAS No: 143614-35-1
Price: $318/10mg
5,6,7,3',4'-Pentamethoxyflavanone
Catalog No: CFN95394
CAS No: 104193-93-3
Price: $318/5mg
4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No: CFN95407
CAS No: 85644-03-7
Price: $318/5mg
12beta-Acetoxy-7beta-hydroxy-3,11,15,23-tetraoxo-5alpha-lanosta-8,20-dien-26-oic acid
Catalog No: CFN95515
CAS No: 1245946-62-6
Price: $318/5mg
Mahuannin G
Catalog No: CFN95553
CAS No: N/A
Price: $318/5mg