Stevenleaf
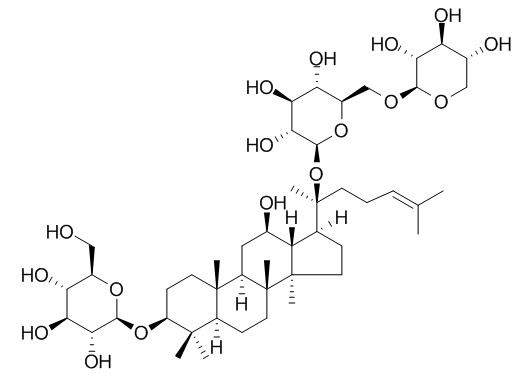
1. Gypenosides (Gyp, Stevenleaf) induce apoptosis in human hepatoma cells through the up-regulation of Bax and Bak, and down-regulation of Bcl-2, release of mitochondrial cytochrome c and activation of caspase cascade.
2. Gypenosides induce ER stress and production of reactive oxygen species and Ca 2+ , change the ratio of Bcl-2 and Bax, followed by the dysfunction of mitochondria, cause cytochrome c release, activation of caspase-3 before leading to apoptosis, these results provide information towards an understanding of the mechanisms by which Gyp induces cell cycle arrest and apoptosis in human tongue cancer cells.
3. Gypenosides can inhibit invasion and migration of human tongue SCC4 cells by down-regulating proteins associated with these processes, resulting in reduced metastasis.
4. Gypenosides imply their remarkable preventative and therapeutic potential in treatment of neurological diseases involving glutamate and oxidative stress.
5. The extensive antioxidant effect of gypenosides may be valuable to the prevention and treatment of various diseases such as atherosclerosis, liver disease and inflammation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Analytical Methods2018, 10(27)
Naunyn Schmiedebergs Arch Pharmacol.2024, 03148-x.
Hindawi J of Food Biochemistry2023, P17:8883860
J. of The Korean Society of Food Culture2017, 144-149
Int J Mol Sci.2024, 25(5):2799.
Antioxidants (Basel).2020, 9(2):E120
Chem Biol Interact.2018, 283:59-74
Toxins (Basel).2021, 13(12):898.
Sci Rep.2017, 7:46299
Mol Divers.2022, s11030-022-10586-3.
Related and Featured Products
Other References Information
Cancer Lett. 2002 Sep 26;183(2):169-78.
Regulation of Bcl-2 family molecules and activation of caspase cascade involved in gypenosides-induced apoptosis in human hepatoma cells.[Pubmed:
12065092]
Gypenosides (Gyp, Stevenleaf ) are triterpenoid saponins contained in an extract from Gynostemma pentaphyllum Makino and reported to induce apoptosis in human hepatoma cells. Our data demonstrated that Gyp-induced apoptotic cell death was accompanied by up-regulation of Bax, Bak and Bcl-X(L), and down-regulation of Bcl-2 and Bad, while it had no effect on the level of Bag-1 protein. Moreover, Gyp treatment caused the release of mitochondrial cytochrome c to cytosol and sequential activation of caspases, including caspase-1, -9 and -3, then leading to cleavage of poly-ADP-ribose polymerase. Furthermore, the Gyp-induced apoptosis was markedly blocked by the broad-spectrum caspase inhibitor, z-VAD-fmk. Taken together, these results suggest that treatment of human hepatoma cells with Gyp induced apoptosis through the up-regulation of Bax and Bak, and down-regulation of Bcl-2, release of mitochondrial cytochrome c and activation of caspase cascade.
Oral Oncol. 2009 Mar;45(3):273-83.
Gypenosides induced G0/G1 arrest via CHk2 and apoptosis through endoplasmic reticulum stress and mitochondria-dependent pathways in human tongue cancer SCC-4 cells.[Pubmed:
18674953 ]
Gypenosides (Gyp, Stevenleaf), a component of Gynostemma pentaphyllum Makino, was selected for examining the effects on the cell viability, cell cycle and induction of apoptosis in human tongue cancer SCC-4 cells. Gyp induced cytotoxicity (decreased the percentage of viable cells) in SCC-4 cells appeared to be associated with induction of cell cycle arrest (G0/G1 arrest), apoptotic cell death based on Gyp induced morphological changes and DNA fragmentation and increased the sub-G1 group in examined SCC-4 cells. In conclusion, Gyp induced ER stress and production of reactive oxygen species and Ca(2+), change the ratio of Bcl-2 and Bax, followed by the dysfunction of mitochondria, caused cytochrome c release, activation of caspase-3 before leading to apoptosis. These results provide information towards an understanding of the mechanisms by which Gyp induces cell cycle arrest and apoptosis in human tongue cancer cells.
Anticancer Res. 2008 Mar-Apr;28(2A):1093-9.
Gypenosides inhibited invasion and migration of human tongue cancer SCC4 cells through down-regulation of NFkappaB and matrix metalloproteinase-9.[Pubmed:
18507059]
Gypenosides (Gyp,Stevenleaf ), components of Gynostemma pentaphyllum Makino, were found to induce suppression of human tongue squamous cell carcinoma SCC4 cell growth and induce apoptosis in response to overexpression of reactive oxygen species, calcium (Ca(+2)) and to decrease mitochondrial membrane potential in vitro. In this study, the effect of Gyp on cell migration and invasion of human tongue SCC4 cells was examined. SCC4 cells treated in vitro with Gyp migrated and invaded less than cells treated with phosphate-buffered saline (PBS) as a control. Gyp inhibited migration and invasion by down-regulating the production of RAS, NFkappaB, COX2, ERK1/2 and MMP-9 relative to PBS only. These results show that Gyp inhibits invasion and migration of human tongue SCC4 cells by down-regulating proteins associated with these processes, resulting in reduced metastasis.
Brain Res. 2006 Aug 2;1102(1):163-74.
Gypenosides protect primary cultures of rat cortical cells against oxidative neurotoxicity.[Pubmed:
16806111]
Gypenosides (GPs, Stevenleaf ) were tested for their ability to protect primary cultures of immature cortical cells against oxidative glutamate toxicity. Results show that GPs significantly up-regulated mRNAs encoding gamma-glutamylcysteine synthetase (gamma-GCS) and glutathione reductase (GR) and enhanced their activities for GSH synthesis as well as recycle. Furthermore, GPs lowered the consumption of GSH through decreased accumulation of intracellular peroxides, leading to an increase in the intracellular GSH content. GPs were also found to prevent lipid peroxidation and reduce the influx of Ca(2+) which routinely follows glutamate oxidative challenge. GPs treatment significantly blocked glutamate-induced decrease in levels of Bcl-2 and increase in Bax, leading to a decrease in glutamate-induced apoptosis. Thus, we conclude that GPs protect cortical cells by multiple antioxidative actions via enhancing intracellular GSH, suppressing glutamate-induced cytosolic Ca(2+) elevation and blocking glutamate-induced apoptosis. The novel role of GPs implies their remarkable preventative and therapeutic potential in treatment of neurological diseases involving glutamate and oxidative stress.
Cancer Biother. 1993 Fall;8(3):263-72.
Protective effect of gypenosides against oxidative stress in phagocytes, vascular endothelial cells and liver microsomes.[Pubmed:
7804367]
The action of gypenosides (GP,Stevenleaf, saponins of Gynostemma pentaphyllum, a Chinese medicinal herb) as an antioxidant was studied using various models of oxidant stress in phagocytes, liver microsomes and vascular endothelial cells. The results show that GP decreased superoxide anion and hydrogen peroxide content in human neutrophils and diminished chemiluminescent oxidative burst triggered by zymosan in human monocytes and murine macrophages. An increase of lipid peroxidation induced by Fe2+/cysteine, ascorbate/NADPH or hydrogen peroxide in liver microsomes and vascular endothelial cells was inhibited by GP. It was also found that GP protected biomembranes from oxidative injury by reversing the decreased membrane fluidity of liver microsomes and mitochondria, increasing mitochondrial enzyme activity in vascular endothelial cells and decreasing intracellular lactate dehydrogenase leakage from these cells. The extensive antioxidant effect of GP may be valuable to the prevention and treatment of various diseases such as atherosclerosis, liver disease and inflammation.
(1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No: CFN95219
CAS No: 1393342-06-7
Price: $413/5mg
Ternatumoside II
Catalog No: CFN95366
CAS No: 1473419-87-2
Price: $318/5mg
5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No: CFN95376
CAS No: 74064-17-8
Price: $318/10mg
(Z)-3,11-dimethy-7-methylene-9,14-epoxy-1,6,10-dodecatrien-3-ol
Catalog No: CFN95402
CAS No: 1392202-57-1
Price: $318/10mg
Benzyl beta-D-glucopyranoside
Catalog No: CFN95427
CAS No: 4304-12-5
Price: $318/10mg
Kaempferol 3,5-O-diglucoside
Catalog No: CFN95487
CAS No: 205103-97-5
Price: $318/10mg
2',4'-Dihydroxychalcone
Catalog No: CFN95493
CAS No: 1776-30-3
Price: $318/20mg
Hosenkoside L
Catalog No: CFN95520
CAS No: 161016-50-8
Price: $318/10mg
12-Acetoxy ganoderic acid D
Catalog No: CFN95535
CAS No: N/A
Price: $318/5mg
Methyl ganoderate B
Catalog No: CFN95551
CAS No: 81907-65-5
Price: $413/5mg



