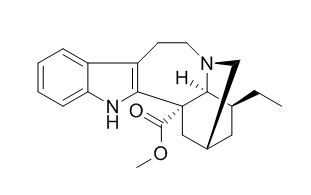
Coronaridine
Coronaridine is an antitumor agent. Coronaridine congeners inhibit hα3β4 AChRs by blocking the ion channel's lumen and probably by additional negative allosteric mechanisms by interacting with a series of non-luminal sites.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceuticals (Basel).2024, 17(9):1130.
BMC Complement Altern Med.2017, 17(1):393
Int Immunopharmacol.2023, 123:110572.
Separations2023, 10(7), 411.
Int J Mol Sci.2018, 19(9):E2681
Plant Biotechnol (Tokyo).2024, 41(3):267-276.
Food Chem.2024, 446:138870.
Foods.2022, 11(12):1708.
Integr Med Res.2024, 13(1):101025.
Regen Biomater.2023, 10:rbad077.
Related and Featured Products
Int J Biochem Cell Biol. 2015 Aug;65:81-90.
Coronaridine congeners inhibit human α3β4 nicotinic acetylcholine receptors by interacting with luminal and non-luminal sites.[Pubmed:
26022277]
To characterize the interaction of Coronaridine congeners with human (h) α3β4 nicotinic acetylcholine receptors (AChRs), structural and functional approaches were used.
METHODS AND RESULTS:
The Ca(2+) influx results established that Coronaridine congeners noncompetitively inhibit hα3β4 AChRs with the following potency (IC50's in μM) sequence: (-)-ibogamine (0.62±0.23)∼(+)-catharanthine (0.68±0.10)>(-)-ibogaine (0.95±0.10)>(±)-18-methoxyCoronaridine [(±)-18-MC] (1.47±0.21)>(-)-voacangine (2.28±0.33)>(±)-18-methylaminoCoronaridine (2.62±0.57 μM)∼(±)-18-hydroxyCoronaridine (2.81±0.54)>(-)-noribogaine (6.82±0.78). A good linear correlation (r(2)=0.771) between the calculated IC50 values and their polar surface area was found, suggesting that this is an important structural feature for its activity. The radioligand competition results indicate that (±)-18-MC and (-)-ibogaine partially inhibit [(3)H]imipramine binding by an allosteric mechanism. Molecular docking, molecular dynamics, and in silico mutation results suggest that protonated (-)-18-MC binds to luminal [i.e., β4-Phe255 (phenylalanine/valine ring; position 13'), and α3-Leu250 and β4-Leu251 (leucine ring; position 9')], non-luminal, and intersubunit sites. The pharmacophore model suggests that nitrogens from the ibogamine core as well as methylamino, hydroxyl, and methoxyl moieties at position 18 form hydrogen bonds.
CONCLUSIONS:
Collectively our data indicate that Coronaridine congeners inhibit hα3β4 AChRs by blocking the ion channel's lumen and probably by additional negative allosteric mechanisms by interacting with a series of non-luminal sites.
Genet Mol Biol. 2013 Mar;36(1):105-10.
Cytotoxicity and genotoxicity of coronaridine from Tabernaemontana catharinensis A.DC in a human laryngeal epithelial carcinoma cell line (Hep-2).[Pubmed:
23569415]
Such as heyneanine, Coronaridine and voacangine.
METHODS AND RESULTS:
The aim of present study was firstly to screen the cytotoxic activity of the indole alkaloids heyneanine, Coronaridine and voacangine against HeLa (human cervix tumor), 3T3 (normal mouse embryo fibroblasts), Hep-2 (human laryngeal epithelial carcinoma) and B-16 (murine skin) cell lines by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); and secondly to analyze the apoptotic activity, cell membrane damage and genotoxicity of the compound that showed the best cytotoxic activity against the tumor cell lines tested. Coronaridine was the one that exhibited greater cytotoxic activity in the laryngeal carcinoma cell line Hep-2 (IC50 = 54.47 μg/mL) than the other alkaloids tested (voacangine IC50 = 159.33 g/mL, and heyneanine IC50 = 689.45 μg/mL). Coronaridine induced apoptosis in cell lines 3T3 and Hep-2, even at high concentrations. The evaluation of genotoxicity by comet assay showed further that Coronaridine caused minimal DNA damage in the Hep-2 tumor cell line, and the LDH test showed that it did not affect the plasma membrane.
CONCLUSIONS:
These results suggest that further investigation of Coronaridine as an antitumor agent has merit.