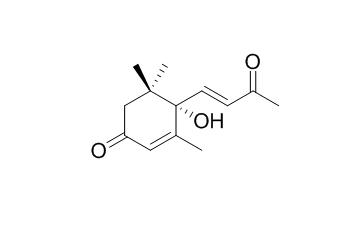
Dehydrovomifoliol
Dehydrovomifoliol could be a marker of Polish heather honey. Dehydrovomifoliol exhibits moderate acetylcholinesterase (AChE) inhibitory activities. Dehydrovomifoliol shows significant cytotoxic activities against three human cancer cell lines, namely, HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma cells, and the IC(50) values in the range 3.7-8.1 microM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Nat Med.2017, 71(2):457-462
J Am Soc Mass Spectrom.2021, 32(5):1205-1214.
Applied Biological Chemistry2021, 64(4)
Journal of Third Military Medical University2018, 40(12):1073-1078
J Colloid Interface Sci.2024, 662:760-773.
Int J Mol Sci.2024, 25(15):8101.
Exp Parasitol.2015, 153:160-4
J Nat Prod.2022, doi: 10.1021
Polytechnic University of Catalonia2017, 105826
Appl. Sci.2025, 15(1), 247
Related and Featured Products
Chem Pharm Bull (Tokyo). 2010 Sep;58(9):1236-9.
Anticholinesterase and antioxidant constituents from Gloiopeltis furcata.[Pubmed:
20823607]
Activity-directed isolation of the ethyl acetate, methylene chloride and n-hexane fractions of Gloiopeltis furcata resulted in the isolation of 18 compounds.
METHODS AND RESULTS:
Their structures were elucidated as 2-(3-hydroxy-5-oxotetrahydrofuran-3-yl)acetic acid (1), glutaric acid (2), succinic acid (3), nicotinic acid (4), (E)-4-hydroxyhex-2-enoic acid (5), cholesterol (6), 7-hydroxycholesterol (7), uridine (8), glycerol (9), 5-(hydroxymethyl)-2-methoxybenzene-1,3-diol (10), (5E,7E)-9-oxodeca-5,7-dienoic acid (11), (Z)-3-ethylidene-4-methylpyrrolidine-2,5-dione (12), Dehydrovomifoliol (13), loliolide (14), cholesteryl stearate (15), palmitic acid (16), cis-5,8,11,14,17-eicosapentaenoic acid (17) and alpha-linolenic acid (18) on the basis of spectroscopic and chemical evidences. Their anticholinesterase and antioxidant activities were evaluated via inhibitory activities on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) as well as scavenging activities on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and peroxynitrite (ONOO(-)).
CONCLUSIONS:
All isolated compounds (1-18) exhibited moderate AChE inhibitory activities with IC(50) values ranging from 1.14-12.50 microg/ml, whereas 1, 7, 9, 17, and 18 showed mild BChE inhibitory activities with IC(50) values ranging from 5.57-15.89 microg/ml.
Although most of the compounds isolated were lacking the scavenging activity on DPPH radical and ONOO(-), 5 and 10 showed good DPPH radical scavenging activity, and 5, 10, and 16 showed potent ONOO(-) scavenging activity.
Chem Pharm Bull (Tokyo). 2009 Apr;57(4):408-10.
Two new sesquiterpenoids from Solanum lyratum with cytotoxic activities.[Pubmed:
19336938]
METHODS AND RESULTS:
Two new sesquiterpenoids, lyratol C (1) and lyratol D (2), together with two known sesquiterpenoids, Dehydrovomifoliol (3) and blumenol A (4), were isolated from the whole plant of Solanum lyratum. Their structures were established by spectroscopic analyses.
CONCLUSIONS:
In vitro, the four compounds showed significant cytotoxic activities against three human cancer cell lines, namely, HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma cells, and gave IC(50) values in the range 3.7-8.1 microM.
J Agric Food Chem. 2014 Apr 2;62(13):2973-81.
Chemometrics as a tool of origin determination of Polish monofloral and multifloral honeys.[Pubmed:
24641200 ]
The aim of this study was to evaluate the application of chemometrics studies to determine the botanical origin of Polish monofloral honeys using NMR spectroscopy.
METHODS AND RESULTS:
Aqueous extracts of six kinds of honeys, namely, heather (Calluna vulgaris L.), buckwheat (Fagopyrum esculentum L), lime (Tilia L), rape (Brassica napus L. var. napus), acacia (Acacia Mill.), and multifloral ones, were analyzed. Multivariate chemometric data analysis was performed using principal component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA). Chemometric analysis supported by pollen analysis revealed the incorrect classification of acacia honeys by the producers. Characteristic motives for each honey were identified, which allowed chemical profiles of tested honeys to be built.
CONCLUSIONS:
Thus, phenylacetic acid and Dehydrovomifoliol (4-hydroxy-4-[3-oxo-1-butenyl]-3,5,5-trimethylcyclohex-2-en-1-one) were proposed to be markers of Polish heather honey. Formic acid and tyrosine were found to be the most characteristic compounds of buckwheat honey, whereas 4-(1-hydroxy-1-methylethyl)cyclohexane-1,3-dienecarboxylic acid was confirmed as a marker of lime honey.