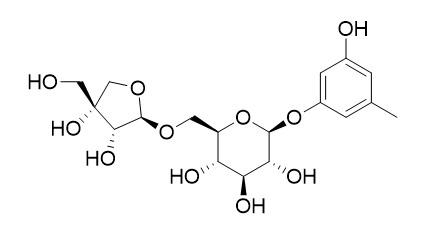
Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside
Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside has antioxidative activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Indian J Pharm Sci.2022, 84(3):144-151
Acta Edulis Fungi2020, 27(02):63-76.
PLoS One.2022, 17(6):e0268505.
BMC Complement Med Ther. 2020, 20(1):94.
Food Sci Nutr.2023, 00:1-10.
Mol Pharm.2018, 15(8):3285-3296
Talanta.2022, 249:123645.
J Appl Pharm Sci.2022, 12(04):044-053
Journal of Food Composition and Analysis2021, 100:103905.
J Ethnopharmacol.2017, 197:157-164
Related and Featured Products
Chem Pharm Bull (Tokyo). 2005 Aug;53(8):1065-7.
Antioxidative phenols and phenolic glycosides from Curculigo orchioides.[Pubmed:
16079552 ]
METHODS AND RESULTS:
A new orcinol glucoside, orcinol-1-O-beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranoside (Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside,3), was isolated from the rhizomes of Curculigo orchioides GAERTN., together with seven known compounds: orcinol glucoside (1), orcinol-1-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranoside (2), curculigoside (4), curculigoside B (5), curculigoside C (6), 2,6-dimethoxyl benzoic acid (7), and syringic acid (8). The structures of these compounds were elucidated using spectroscopic methods. The antioxidant activities of these isolated compounds were evaluated by colorimetric methods based on their scavenging effects on hydroxyl radicals and superoxide anion radicals, respectively.
CONCLUSIONS:
All the compounds showed potent antioxidative activities and the structure-activity relationship is discussed.
J Pharm Biomed Anal. 2015 Jan;102:236-45.
Qualitative and quantitative analysis on chemical constituents from Curculigo orchioides using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry.[Pubmed:
25305598]
A rapid ultra-high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC-ESI-Q-TOF/MS) method was developed for qualitative and quantitative determination of constituents in the rhizome of Curculigo orchioides.
METHODS AND RESULTS:
Qualitative analysis was performed on a Waters ACQUITY UHPLC @ HSS T3 column (1.8 μm 100 × 2.1mm) using gradient elution with mobile phase of 0.1% formic acid and acetonitrile. Quantitative analysis was performed on an Agilent ZORBAX Eclipse plus C18 column (1.7 μm 100 × 2.1mm) using gradient elution with mobile phase of 0.1% acetic acid and acetonitrile for at least 20 min. Quadrupole TOF/MS in either full scan mode or extracted ion mode was used for qualitative and quantitative analysis of the constituents. According to the mass spectrometric fragmentation mechanism and UHPLC-ESI-Q-TOF-MS data, chemical structures of 45 constituents in the rhizome of Curculigo orchioides, including 19 phenols and phenolic glycosides, 16 lignans and lignan glycosides, 8 triterpenoid saponins, one flavone and one sesquiterpene, were identified tentatively on-line without the time-consuming process of isolation. In addition, 8 phenolic glycosides including 5-hydroxymethylfurfural (HMF), 2-hydroxy-5-(2-hydroxyethyl) phenyl-β-D-glucopyranoside (HPG), anacardoside (ACD), orcinol glucoside (OGD), orcinol-1-O-β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranoside (Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside,OAG), 2,6-dimethoxybenzoic acid (DBA), curculigoside (CUR) and curculigine A (CCL) were quantitated in 11 collected samples and 10 commercial samples from different providers.
CONCLUSIONS:
The results show that UHPLC-ESI-Q-TOF-MS is a viable method for analysis and quality evaluation of the constituents from the rhizome of Curculigo orchioides.