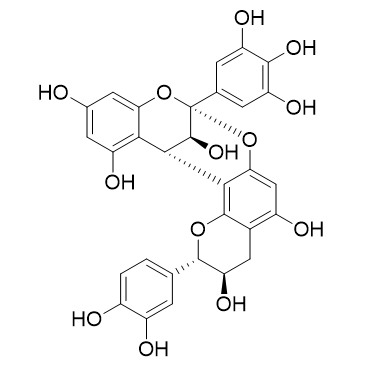
Ephedrannin D1
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2016, 191:81-90
Journal of Mushroom2023, 21(4):215-221.
Molecules.2024, 29(11):2626.
Egyptian Pharmaceutical Journal2024, epj_205_23.
J Biomol Struct Dyn.2022, 1-21.
Acta Pharm Sin B.2024, 14(4):1772-1786.
J Nat Sc Biol Med2019, 10(2):149-156
Int Immunopharmacol.2019, 71:22-31
Journal of Phytopathology2021, 169,Issue11-12.
Molecules.2023, 28(4):1526.
Related and Featured Products
Molecules . 2013 May 6;18(5):5172-5189.
A-type proanthocyanidins from the stems of Ephedra sinica (Ephedraceae) and their antimicrobial activities[Pubmed:
23648595]
Phytochemical investigation of the n-BuOH-soluble fraction of the EtOH extract of the herbaceous stems of Ephedra sinica, which is known as Ephedrae Herba in Traditional Chinese Medicine, led to the isolation and identification of 12 A-type proanthocyanidins, containing five dimers, two trimers and five tetramers [i.e., (+)-epigallocatechin-(2α→O→7,4α→8)-(-)-catechin, named ephedrannin D₁, a dimer; epigallocatechin-(2α→O→7,4α→8)-epigallocatechin-(4α→8)-catechin (ephedrannin Tr₁), a trimer; and epigallocatechin-(2α→O→7,4α→8)-epigallocatechin-(4α→8)-epigallocatechin-(2α→O→7,4α→8)-gallocatechin, named ephedrannin Te1, a tetramer). Tetramers composed of gallocatechin are reported for the first time in Ephedraceae. Catechin, epicatechin, gallocatechin, epigallocatechin and four known dimers were also isolated. The structures were elucidated by extensive spectroscopic analysis. The absolute configurations of the 4α linkages, which were confirmed by NOESY and CD experiments, are the outstanding characteristic of most of these isolated A-type proanthocyanidins. The antimicrobial activities of these compounds were tested by measuring the minimum inhibitory concentrations (MIC) against bacteria (both Gram positive and Gram negative) and fungi, and were found to be in the range of 0.00515-1.38 mM. Compounds 6, 8, 10 and 11 exhibited moderate antimicrobial activities against Canidia albicans.