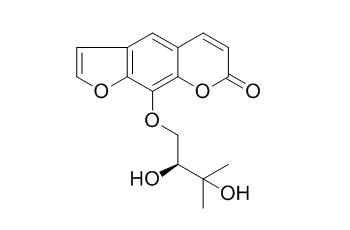
(-)-Heraclenol
(-)-Heraclenol shows in vitro anti-plasmodial activity against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum.(-)-Heraclenol has moderate antibacterial and antifungal activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Sci Nutr.2023, 11(9):5532-5542.
Proc. Sci. Math.2024, V25:108-123
Biomed Pharmacother.2021, 144:112300.
Plants (Basel).2020, 9(11):1422.
Molecules.2017, 22(3)
AMB Express2020. 10(1):126.
FEBS J.2022, 10.1111:febs.16676.
African J. Agricultural Research 2017, 12(13):1164-1168
EXCLI J.2023, 22:482-498.
Planta Med.2018, 84(15):1101-1109
Related and Featured Products
Current Science, 2011, 100(11):1706-1711.
Microbial transformation of (+)-heraclenin by Aspergillus niger and evaluation of its antiplasmodial and antimicrobial activities[Reference:
WebLink]
METHODS AND RESULTS:
Microbial transformation of (+)-heraclenin (1) by Aspergillus niger was studied in growth media to assess its antiplasmodial and antimicrobial activities. It was transformed to (-)-Heraclenol (2) as the sole product in a stereospecific manner. The in vitro antiplasmodial activity of compounds 1 and 2 was tested with chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum. Further, the in vitro antibacterial activity of 1 and 2 against three Grampositive bacteria, Bacillus subtilis, Bacillus sphaericus and Staphylococcus aureus, and three Gram-negative bacteria, Pseudomonas aeruginosa, Escherichia coli and Chromobacterium violaceum was analysed using agar-plate diffusion assay. The same method was employed for the evaluation of antifungal activity against five pathogenic strains of fungi, A. niger, Rhizopus oryzae, Aspergillus flavus, Candida albicans and Saccharomyces cerevisiae.
CONCLUSIONS:
Both furanocoumarins 1 and 2 displayed significant levels of antiplasmodial and moderate levels of antimicrobial activities against the tested pathogenic strains. Compound 2 exhibited two-fold less potent antiplasmodial activity (IC50 = 6.0 μg/ml) than the parent compound 1 (IC50 = 2.5 μg/ml), whereas no difference was observed in the antimicrobial activity of both furanocoumarins. The oxirane ring was found to be beneficial in terms of antiplasmodial activity.