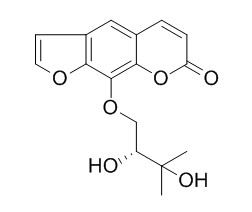
Heraclenol
Heraclenol is a germination inhibitor in the parsley seeds. It has anti-inflammatory properties against the ear edema in mice produced by TPA. Heraclenol and heraclenin inhibit the proliferation of melanoma cells and cell cycle at G2/M at concentrations of 0.1-1.0 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Cell Mol Med.2020, 24(21):12308-12317.
Nutr Cancer.2023, 75(1):376-387.
Plant Biotechnol (Tokyo).2024, 41(3):267-276.
Natural Product Sciences2024, 30(4):254-261.
Food Res Int.2020, 128:108778
Molecules.2021, 26(9):2802.
Cosmetics2021, 8(3),91.
Front Endocrinol (Lausanne).2020, 11:568436.
Front Pharmacol.2021, 12:744624.
Molecules.2020, 25(21):5091.
Related and Featured Products
Phytochemistry,1978,17(1):158-9.
The coumarin heraclenol as a growth inhibitor in parsley seeds.[Reference:
WebLink]
METHODS AND RESULTS:
By the aid of germination assay with lettuce seeds, the germination inhibitors in the parsley seeds were investigated. One of the inhibitors was revealed to be Heraclenol.
Planta Med. 2000 Apr;66(3):279-81.
Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model.[Pubmed:
10821059]
METHODS AND RESULTS:
From the aerial parts of Decatropis bicolor, heraclenin (1), seselin (2), psoralen (3), imperatorin (4), skimmianine (5), and Heraclenol (6), were isolated. This is the first time that coumarin-like compounds are isolated from Decatropis genus. The anti-inflammatory properties of compounds 1-6 were examined against the ear edema in mice produced by TPA.
CONCLUSIONS:
The results suggest that the anti-inflammatory activity of each compound depends of its individual substitution on the aromatic ring rather than the coumarin skeleton itself.
Photochem Photobiol. 2013 Sep-Oct;89(5):1216-25.
In vitro and in vivo antiproliferative effect of a combination of ultraviolet-A and alkoxy furocoumarins isolated from Umbelliferae medicinal plants, in melanoma cells.[Pubmed:
23802687]
METHODS AND RESULTS:
We examined the effects of six furocoumarins with alkoxy groups at the C-5 or C-8 position isolated from Umbelliferae medicinal plants on cell proliferation, and their mechanisms of action against B16F10 melanoma cells or in melanin-possessing hairless mice implanted with B16F10 cells, under UVA irradiation. Three furocoumarins with an alkoxy group at C-5, isoimperatorin (1), oxypeucedanin (2) and oxypeucedanin hydrate (3), showed antiproliferative activity and caused G2/M arrest at concentrations of 0.1-10.0 μm. Furthermore, three furocoumarins with an alkoxy group at C-8, imperatorin (4), heraclenin (5) and Heraclenol (6), inhibited the proliferation of melanoma cells and cell cycle at G2/M at concentrations of 0.1-1.0 μm. UVA plus 1, 2, 3, 4 and 6 reduced tumor growth and final tumor weight in B16F10-bearing mice at a dose of 0.3, 0.5 or 1.0 mg kg(-1) (intraperitoneal injection). UVA plus 1, 3 and 6 increased Chk1 phosphorylation and reduced cdc2 (Thr 161) phosphorylation in melanoma cells.
CONCLUSIONS:
We suggest that the antitumor actions of UVA plus furocoumarins with an alkoxy group at C-5 or C-8 were due to G2/M arrest of the cell cycle by an increase in phosphor-Chk1 and decrease in phospho-cdc2.
PLoS One. 2014 Oct 14;9(10):e108713.
Isolation, cytotoxicity evaluation and HPLC-quantification of the chemical constituents from Prangos pabularia.[Pubmed:
25314269]
Phytochemical analysis of the dichloromethane:methanol (1:1) extract of root parts of Prangos pabularia led to the isolation of twelve cytotoxic constituents, viz., 6-hydroxycoumarin (1), 7-hydroxycoumarin (2), Heraclenol-glycoside (3), xanthotoxol (4), Heraclenol (5), oxypeucedanin hydrate (6), 8-((3,3-dimethyloxiran-2-yl)methyl)-7-methoxy-2H-chromen-2-one (7), oxypeucedanin hydrate monoacetate (8), xanthotoxin (9), 4-((2-hydroxy-3-methylbut-3-en-1-yl)oxy)-7H-furo[3,2-g]chromen-7-one (10), imperatorin (11) and osthol (12).
METHODS AND RESULTS:
The isolates were identified using spectral techniques in the light of literature. 3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity screening of the isolated constituents was carried out against six human cancer cell lines including lung (A549 and NCI-H322), epidermoid carcinoma (A431), melanoma (A375), prostate (PC-3) and Colon (HCT-116) cell lines. Osthol (12) exhibited the highest cytotoxicity with IC50 values of 3.2, 6.2, 10.9, 14.5, 24.8, and 30.2 µM against epidermoid carcinoma (A431), melanoma (A375), lung (NCI-H322), lung (A549), prostate (PC-3) and colon (HCT-116) cell lines respectively. Epidermoid carcinoma cell line A431 was sensitive to most of the compounds followed by lung (A549) cancer cell line. Finally a simple and reliable HPLC method was developed (RP-HPLC-DAD) and validated for the simultaneous quantification of these cytotoxic constituents in Prangos pabularia. The extract was analyzed using a reversed-phase Agilent ZORBAX eclipse plus column C18 (4.6×250 mm, 5 µm) at 250 nm wavelength using a gradient water-methanol solvent system at a flow rate of 0.8 ml/min.
CONCLUSIONS:
The RP-HPLC method is validated in terms of recovery, linearity, accuracy and precision (intra and inter-day validation). This method, because of shorter analysis time, makes it valuable for the commercial quality control of Prangos pabularia extracts and its future pharmaceutical preparations.
Nat Prod Commun. 2012 Oct;7(10):1327-30.
Phytotoxic furanocoumarins from the shoots of Semenovia transiliensis.[Pubmed:
23157001]
METHODS AND RESULTS:
A number of furanocoumarin compounds isolated from S. transiliensis shoots were phytotoxic to both test species. These included psoralen, isopsoralen, heratomin, isopentenyloxyisobergapten, imperatorin, bergapten, xanthotoxin, heraclenin, and Heraclenol.
CONCLUSIONS:
All the active secondary metabolites isolated from the shoots of S. transiliensis were furanocoumarins.