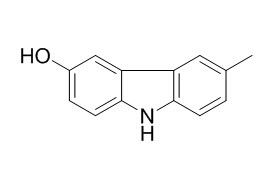
Glycozolinine
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Appl. Sci.2021, 11(19),9343.
Front Pharmacol.2021, 12:615157.
Front Pharmacol.2021, 12:765521.
Front Plant Sci.2021, 12:673337.
Talanta Open2023, 7:100227
In Vitro Cellular & Developmental Biology - Plant2022, 58:972-988.
Phytomedicine.2022, 100:154085.
VNU Journal of Science2023, No. 20.
Int J Mol Sci.2022, 23(23):15213.
Korean Journal of Pharmacognosy.2015, 46(4):352-364
Related and Featured Products
Other References Information
Phytochemistry Volume 22, Issue 4, 1983, Pages 1064-1065
Glycozolinine, a carbazole derivative from Glycosmis pentaphilla[Reference:
WebLink]
A new carbazole derivative, Glycozolinine, was isolated from the seeds of Glycosmis pentaphylla. From physical and chemical evidence its structure is 6-hydroxy-3-methylcarbazole.
Tetrahedron Volume 71, Issue 21, 27 May 2015, Pages 3485–3490
Total synthesis of glycomaurrol and eustifoline-C by DIBAL-H promoted reductive ring opening of pyrano[2,3-c]carbazoles[Reference:
WebLink]
The palladium-catalyzed construction of the carbazole framework provides an efficient access to 3-hydroxy-6-methylcarbazole (Glycozolinine) (5). Regioselective annulation of a pyran ring at Glycozolinine (5) using either a C5- or a C10-building block leads to the pyrano[2,3-c]carbazole alkaloids glycomaurin (5,6-pyranoglycozoline, eustifoline-A) (2) and eustifoline-B (4), respectively.