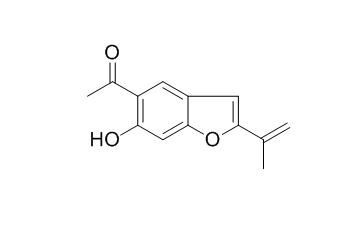
Euparin
Euparin exhibits a moderate antioxidant activity, it also
exhibits antifungal activity against Trichophyton mentagrophytes. Euparin has antiviral activity, it exerts its effect during the early events of the replication cycle, from the virus adsorption to cells up to the first twenty minutes after infection.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
bioRxiv-Pharm.&Toxi.2022, 2022.481203.
The Korea Journal of Herbology2019, 34(2):25-32
Int J Mol Sci.2022, 23(24):16000.
J Cell Mol Med.2020, 24(21):12308-12317.
Reprod Toxicol.2020, 96:1-10.
ACS Synth Biol.2022, doi: 10.1021.
Food Sci Biotechnol.2021, 30(2):217-226.
Pharmaceutics.2023, 15(6):1771.
ACS Synth Biol.2022, 11(10):3296-3304.
The Catharanthus Genome2022,35-83.
Related and Featured Products
Evid Based Complement Alternat Med. 2013;2013:402364.
Antipoliovirus Activity of the Organic Extract of Eupatorium buniifolium: Isolation of Euparin as an Active Compound.[Pubmed:
23956770]
METHODS AND RESULTS:
The antiviral activity of the organic extract (OE) of Eupatorium buniifolium against poliovirus type 1 was determined by in vitro assays with an effective concentration 50 (EC50) of 23.3 ± 3.3 μg/mL.
Bioassay-guided fractionation of the OE allowed the isolation of an active principle that was identified by spectroscopic methods ((1)H- and (13)C-NMR, EI-MS, UV, and IR spectroscopy) as the benzofuran Euparin. The plaque reduction assay in Vero cells was used to assess the antiviral activity of Euparin against poliovirus types 1, 2, and 3 with EC50 values of 0.47, 0.12, and 0.15 μg/mL, respectively. Moreover, this compound showed high selectivity indexes of 284.9, 1068, and 854.7, respectively. In order to identify the mechanism by which Euparin exerts its antiviral activity, the virucidal effect, the pretreatment of Vero cells, and the time of action on one viral replication cycle were evaluated.
CONCLUSIONS:
Results obtained demonstrated that Euparin exerts its effect during the early events of the replication cycle, from the virus adsorption to cells up to the first twenty minutes after infection. This is the first report on the presence of Euparin in E. buniifolium and its antiviral activity.
J Nat Prod. 1996 Mar;59(3):323-6.
Phytogrowth-inhibitory and antifungal constituents of Helianthella quinquenervis.[Pubmed:
8882437]
METHODS AND RESULTS:
Investigation on the roots of Helianthella quinquenervis (Hook.) A. Gray (Asteraceae), led to the isolation of one new benzofuran (6-methoxy-tremetone (1)) and a new prenylacetophenone (4-beta-D-(glucopyranosyloxy)-3-[3-methoxy-trans-isopenten-1 -yl] acetophenone (3)). In addition, 6-hydroxy-3-methoxytremetone (2), encecalin (6), Euparin (5), demethylencecalin (4), and angelic acid were obtained. Structural assignments of the isolated compounds were based on spectroscopic and spectrometric analysis.
CONCLUSIONS:
Natural products 1-4 showed marginal cytotoxicity against three human tumor cell lines [MCF-7, A-549, and HT-29]. Compounds 4 and 6 inhibited the radicle growth of Amaranthus hypochondriacus and Echinochloa crusgalli. Furthermore, substances 4-6 exhibited antifungal activity against Trichophyton mentagrophytes.
Nat Prod Res. 2017 Jun;31(11):1325-1328.
In vitro evaluation of cytotoxic activity of the ethanol extract and isolated compounds from the corms of Liatris spicata (L.) willd on HepG2.[Pubmed:
27712097]
METHODS AND RESULTS:
Investigation of the ethanol extract of the corms of Liatris spicata (L.) willd led to the isolation of two sterols: stigmasterol and its 3-O-glucoside, a triterpene: obtusifoliyl acetate, two benzofurans: Euparin and 6-hydroxy-3-methoxytremetone, three phenolic acids: protocatechuic, vanillic and ferulic acid and a sesquiterpene lactone igalan. The structures of the isolated compounds were established on the basis of physicochemical properties and spectral analysis (IR, EI/MS, 1H NMR and 13C NMR).
CONCLUSIONS:
The ethanol extract and its isolated compounds evidenced cytotoxic activities against human liver cancer cell line (HepG2), where igalan showed the highest potency (3.83 ± 0.043) μg/mL, its effect was comparable to that of the standard drug doxorubicin® (3.73 ± 0.036) μg/mL.