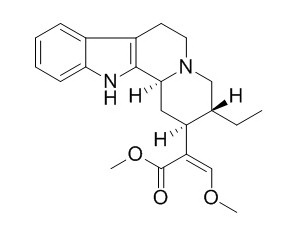
Dihydrocorynantheine
Dihydrocorynantheine shows pronounced activity against Leishmania major promastigotes (IC50 at the micromolar level) but no significant in vitro antiplasmodial activity (against chloroquinesensitive Plasmodium falciparum). Dihydrocorynantheine exhibits significant vasodilating activity against phenylephrine-induced contraction in rat thoracic aorta rings (IC(50) = 6.73 μg/mL), it also shows weak inhibitory action on KCl-induced contraction.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2023, 24(24):17589.
Antioxidants2022, 11(2),234.
Mol Med Rep.2024, 29(2):26.
Biochem Biophys Res Commun.2020, 522(1):40-46
Current Topics in Nutraceutical Research2021, 19(1),p90-105.
Life Sci.2019, 216:259-270
Front Pharmacol.2016, 7:460
Inflammation.2022, 45(6):2529-2543.
Pak J Pharm Sci.2023, 36(1):51-57.
Evid Based Complement Alternat Med.2021, 2021:6687513.
Related and Featured Products
Planta Medica, 2014 , 66 (6) :531-6.
Leishmanicidal, Antiplasmodial and Cytotoxic Activity of Indole Alkaloids from Corynanthe pachyceras.[Reference:
WebLink]
Five indole alkaloids, corynantheidine, corynantheine, Dihydrocorynantheine, -yohimbine and corynanthine were isolated from bark of Corynanthe pachyceras K. Schum. (Rubiaceae).
METHODS AND RESULTS:
The structures were established by spectroscopic methods, inlcuding previously unreported assignment of all 1 HNMR resonances by COSY and NOESY experiments. These and related alkaloids showed pronounced activity against Leishmania major promastigotes (IC50 at the micromolar level) but no significant in vitro antiplasmodial activity (against chloroquinesensitive Plasmodium falciparum).
CONCLUSIONS:
Cytotoxicity assessed with drug sensitive KB-3-1 and multidrug-resistant KB-V1 cell lines was low; the alkaloids are apparently not substrates for the Pglycoprotein (P-170) efflux pump.
J Nat Prod. 2011 Jan 28;74(1):12-5.
Macrophyllionium and macrophyllines A and B, oxindole alkaloids from Uncaria macrophylla.[Pubmed:
21070010 ]
METHODS AND RESULTS:
An unusual oxindole alkaloid inner salt, macrophyllionium (1), and a pair of new tetracyclic oxindole alkaloids, macrophyllines A (2) and B (3), together with six known alkaloids, were isolated from the aerial parts of Uncaria macrophylla.
CONCLUSIONS:
Corynantheidine (8) exhibited moderate cytotoxicity against HL-60 and SW480 cells with IC(50) values of 13.96 and 23.28 μM, respectively. Dihydrocorynantheine (9) exhibited significant vasodilating activity against phenylephrine-induced contraction in rat thoracic aorta rings (IC(50) = 6.73 μg/mL). In addition, compounds 2, 6, and 9 showed weak inhibitory action on KCl-induced contraction.