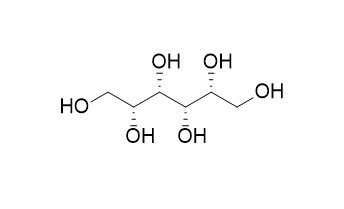
D-Iditol
D-Iditol may have potential antitumour activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Exp Parasitol.2018, 194:67-78
STAR Protoc.2024, 5(2):102990.
J Ethnopharmacol.2017, 196:75-83
Phytomedicine.2024, 128:155527.
Sci Rep. 2017, 12953(7)
Appl Biochem Biotechnol.2020, 190(2):732-744
J Ginseng Res.2020, 44(4):611-618.
Pathogens.2018, 7(3):E62
Front Immunol.2024, 15:1423776.
Sci Rep.2015, 5:13194
Related and Featured Products
J Biosci Bioeng . 1999;87(4):548-550.
Production of D-iditol from D-sorbose by Rhodotolura rubra RY10 isolated from miso paste[Pubmed:
16232515]
The yeast strain RY10 that can convert D-sorbose to D-Iditol was isolated from miso paste and identified as Rhodotolura rubra. The cells grown on D-fructose were found to have relatively high conversion potential. Addition of ethanol to the reaction mixture significantly accelerated the conversion rate of D-sorbose to D-Iditol. During the conversion reaction, ethanol was added to the reaction mixture at 48 h intervals to maintain the concentration of ethanol at 1.0%. The final conversion ratios were 82.7%, 95.0%, 93.7%, and 78.0% using washed cells when the concentration D-sorbose were 1.0%, 2.0%, 3.0% and 5.0%, respectively. The product produced from D-sorbose was identified as D-Iditol by high performance liquid chromatography analysis, infrared spectrum, optical rotation and melting point measurements.
Org Lett . 2005 Jun 9;7(12):2305-2308.
2-Ene-1,4-diols by dimerization of terminal epoxides using hindered lithium amides[Pubmed:
15932184]
[reaction: see text] Reaction of hindered lithium amides with readily available (enantiopure) terminal epoxides gives 2-ene-1,4-diols via carbenoid dimerization of the corresponding alpha-lithiated epoxides. D-Mannitol and D-Iditol were synthesized using this method in three steps from (S)-tritylglycidyl ether.