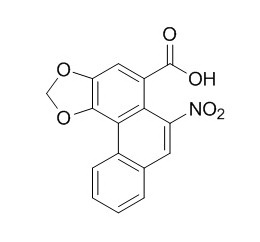
Aristolochic acid B
Aristolochic acid II (Aristolochic acid B,AAII), one of the major components of the carcinogenic plant extract aristolochic acid, is known to be mutagenic and to form DNA adducts in vitro and in vivo, AAII shows more carcinogenic risk than aristolochic acid I, and this may be, at least partly, the result of its increased levels in kidney and plasma.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Evid Based Complement Alternat Med.2015, 2015:165457
Pamukkale Medical Journal2022, 15(4):796-803.
Agronomy2023, 13(9), 2410.
Pharmaceutical Chemistry Journal2019, 52(12):986-991
Saudi Pharm J2020, 10.1016
J Nat Med.2018, 72(3):734-744
Molecules.2021, 26(13):4081.
Front Plant Sci.2018, 9:1424
Revista Brasileira de Farmacognosia2024, 34:1156-1165.
J Sci Food Agric.2018, 98(3):1153-1161
Related and Featured Products
Am J Chin Med. 2008;36(2):425-36.
Production, characterization of a monoclonal antibody against aristolochic acid-B and development of its assay system.[Pubmed:
18457371]
Aristolochic acid-II (Aristolochic acid B, AA-II) conjugated with bovine serum albumin (BSA) was used as an antigen for immunizing BALB/c mice.
METHODS AND RESULTS:
Isolated splenocytes from the immunized mice were fused with an aminopterin-sensitive mouse myeloma cell line, SP2/0-Ag14, to produce hybridoma cells that secreted a monoclonal antibody (MAb) against AA-II. The selected hybridoma was subsequently cloned by limited dilution method. For MAb, the isotype and an estimated dissociation constant (K(D)) of the MAb were determined. The MAb was used to establish an ELISA method. Accuracy and variation assays, as well as determinations of the specificity and sensitivity, were also carried out and the linear range was 0.19-13 microg/ml. The anti-AA-II MAb showed a very high specificity for AA-II and had low cross-reactivities against the other aristolochic acid (AAs) (CR: AA-I, 3.4%; AA-VIIa, 0.86%) or aristololactam-I (AL-I) (CR<0.07%) except AA-IIIa which has 17% of cross activity. Anti-AA-II MAb also showed negligible cross-reactivity (<0.5%) toward other natural compounds with different chemical structures including barbaloin, sennoside A, rutin, glycyrrhizin, caffeic acid etc.
CONCLUSIONS:
This is the first time that an ELISA method was successfully established for the application of anti-AA-II MAb.
Mutat Res. 2012 Mar 18;743(1-2):52-8.
Comparison of the mutagenicity of aristolochic acid I and aristolochic acid II in the gpt delta transgenic mouse kidney.[Pubmed:
22245565 ]
Aristolochic acid (AA) is known to be a potent mutagen and carcinogen. Aristolochic acid I (Aristolochic acid A, AAI) and aristolochic acid II (Aristolochic acid B, AAII), the two major components of AA, differ from each other by a single methoxy group. However, their individual mutagenic characteristics in vivo are unclear.
METHODS AND RESULTS:
In the present study, we compared their DNA adduct formation and mutagenicities in the gpt delta transgenic mouse kidney. The dA-AAI, dG-AAI, dA-AAII and dG-AAII were identified in the kidney two days after intragastric administration of AAI or AAII at 5mg/kg. The concentration of DNA adducts formed by AAII was approximately 2.5-fold higher than that formed by AAI (p<0.05). The mutant frequency induced by AAII was nearly two-fold higher than that induced by AAI (p<0.05) following administration of 5mg/kg AAI or AAII, five times per week for six weeks. Investigation of the mutation spectra showed no statistically significant difference between AAI- and AAII-treated mice (p>0.05). A:T to T:A transversion was the predominant type of mutation in both treated groups, the GC-associated mutation rates, however, differed between the AAI and AAII treatments. The in vivo metabolic pathways of AAI and AAII are different, and this may affect their mutagenicity. In the present study, we measured the levels of AAI and AAII in the kidney and plasma of gpt delta transgenic mice at multiple time points after a single intragastric dose of 1 or 5mg/kg of either component. Our results showed that the levels of AAII in both kidney and plasma were considerably higher than those of AAI (p<0.01).
CONCLUSIONS:
The present study indicated that AAII showed more carcinogenic risk than AAI in vivo, and this may be, at least partly, the result of its increased levels in kidney and plasma.
Chem Res Toxicol. 1991 Sep-Oct;4(5):581-6.
N6-adenyl arylation of DNA by aristolochic acid II and a synthetic model for the putative proximate carcinogen.[Pubmed:
1665354]
Aristolochic acid II (Aristolochic acid B, AAII), one of the major components of the carcinogenic plant extract aristolochic acid, is known to be mutagenic and to form DNA adducts in vitro and in vivo.
METHODS AND RESULTS:
The major fluorescent DNA adduct formed upon xanthine oxidase mediated reduction in the presence of calf thymus (CT-) DNA or deoxyadenosine was isolated by means of preparative HPLC and identified by fluorescence, UV/vis absorbance, and 1H NMR spectroscopy as 7-(deoxy-adenosin-N6-yl)aristolactam II. As a model proximate carcinogen, N-chloroaristolactam II was prepared chemically from aristolactam II, the reduction product of AAII. This model compound was spectroscopically characterized and found to react directly with CT-DNA without any activation, forming the same deoxyadenosine adduct. HPLC analysis with fluorescence monitoring detected this adduct in vivo in the liver DNA of Wistar rats treated orally with AAII.
CONCLUSIONS:
These results confirm the anticipated metabolic activation mechanism of AAII as occurring via a cyclic nitrenium ion.