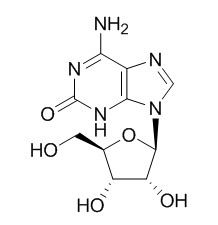
Crotonoside
Crotonoside is one compound of an antitumor and immunity-regulating pharmaceutical composition of traditional Chinese medicine.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2017, 18(12)
Journal of Physiology & Pathology in Korean Medicine.2018, 32(2): 106-112
Antioxidants (Basel).2020, 9(4):326.
Korean J Pain.2021, 34(4):405-416.
Food Funct.2022, 13(23):12105-12120.
Exp Neurobiol.2018, 27(3):200-209
J Nat Sc Biol Med2019, 10(2):149-156
Natural Product Communications2022, 7(3):1-7.
J Ethnopharmacol.2023, 313:116534.
J Pharmaceutical Research Int.2021, 33(41A):275-284.
Related and Featured Products
PAT - WO2010148577
ANTITUMOR AND IMMUNITY-REGULATING PHARMACEUTICAL COMPOSITION OF TRADITIONAL CHINESE MEDICINE, PREPARATION METHOD AND USE FOR SAME[Reference:
WebLink]
An antitumor and immunity-regulating pharmaceutical composition of traditional Chinese medicine, preparation method and use for same. The pharmaceutical composition comprises Semen crotonis pulveratum, Radix Platycodi and Fritillaria with a weight ratio of 1:3:3. Semen crotonis pulveratum contains 8-12% croton oil and 1.47-5.59% Crotonoside, and is prepared by extracting croton kernel, isolating croton oil and croton dregs, and proportionately adding croton oil into croton dregs. Said composition is both curative and highly safe.
Arch Pharm Res. 1994 Apr;17(2):115-8.
Isolation of isoguanosine from Croton tiglium and its antitumor activity.[Pubmed:
10319142]
This paper describes the isolation of isoguanosine(Crotonoside) from Croton tiglium L. and its cytotoxic effect against several tumor cell lines in culture and newly reports that isoguanosine(Crotonoside) has an antitumor activity against implanted S-180 ascitic tumor mice.
METHODS AND RESULTS:
Isoguanosine is effective at the dose of 24 mg/kg/day x 5, with T/C value of 168%. Isoguanosine inhibits the growth of S-180 and Ehrlich solid tumor in mice at the optimal doses of 96 mg/kg/day x 12 and 48 mg/kg/day x 12, with 1-T/C values of 65% and 60%, respectively.
J Phys Chem A. 2013 Jul 18;117(28):5715-25.
Mechanism of the deamination reaction of isoguanine: a theoretical investigation.[Pubmed:
23789717]
METHODS AND RESULTS:
Mechanisms of the deamination reactions of isoguanine (Crotonoside) with H2O, OH(-), and OH(-)/H2O and of protonated isoguanine (isoGH(+)) with H2O have been investigated by theoretical calculations. Eight pathways, paths A-H, have been explored and the thermodynamic properties (ΔE, ΔH, and ΔG), activation energies, enthalpies, and Gibbs energies of activation were calculated for each reaction investigated. Compared with the deamination reaction of isoguanine or protonated isoguanine (isoGH(+)) with water, the deamination reaction of isoguanine with OH(-) shows a lower Gibbs energy of activation at the rate-determining step, indicating that the deamination reaction of isoguanine is favorably to take place for the deprotonated form isoG(-) with water.
CONCLUSIONS:
With the assistance of an extra water, the reaction of isoguanine with OH(-)/H2O, pathways F and H, are found to be the most feasible pathways in aqueous solution due to their lowest Gibbs energy of activation of 174.7 and 172.6 kJ mol(-1), respectively, at the B3LYP/6-311++G(d,p) level of theory.
Biochemistry. 1994 Oct 11;33(40):12119-26.
Isoguanosine substitution of conserved adenosines in the hammerhead ribozyme.[Pubmed:
7918433]
METHODS AND RESULTS:
Isoguanosine(Crotonoside) has been incorporated into a 34-mer hammerhead ribozyme by the solid-phase phosphoramidite method, using an acetamidine base protecting group. The activity of the hammerhead ribozyme when singly mutated to isoguanosine at the adenosine positions 6, 9, and 13 was 1-2-fold less than the wild-type activity. Mutations to 2-aminopurine ribonucleoside at positions 9 and 13 were 5-fold reduced in activity, but that at position 6 was approximately 30-fold reduced.
CONCLUSIONS:
These results support the view that the 6-amino functions of A6, A9, and A13 are not very important for catalysis. The 2-position of A6 tolerates a carbonyl function but not an amino group, whereas A9 and A13 tolerate both functional groups. The tolerance of a 2-amino group at A9 and A13 makes G(anti)/A(anti) Watson-Crick type base mispairing for G12/A9 and A13/G8 unlikely.